Abstract
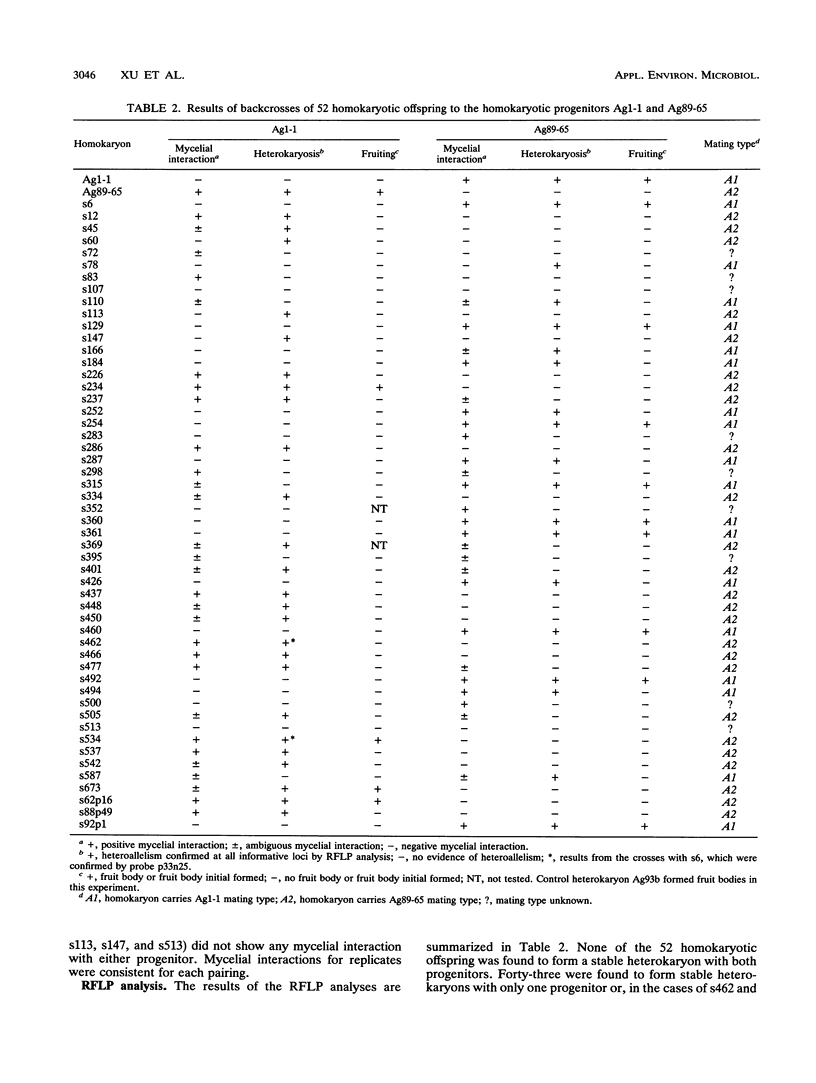
The cultivated mushroom Agaricus bisporus is secondarily homothallic. Most basidia produce two basidiospores, each of which receives two of the four postmeiotic nuclei. Usually, the two packaged nuclei carry compatible mating types. Previous studies suggested that there may be only a single mating type locus in A. bisporus. In this study, we determined whether the mating type segregated as a single Mendelian determinant in a cross marked with 64 segregating molecular markers. To score mating types, each of the 52 homokaryotic offspring from this cross was paired with each of the two progenitor homokaryons. Compatible matings were identified by the formation of genetically stable heterokaryons which were verified by assay of restriction fragment length polymorphisms (RFLPs). Data for screening mycelial interactions on petri plates as well as fruit body formation were compared with the RFLP results. Mating types of 43 of the 52 homokaryotic offspring were determined on the basis of RFLP analysis. Our results indicate (i) there is a segregating mating type gene in A. bisporus, (ii) this mating type gene is on the largest linkage group (chromosome I), (iii) mycelial interactions on petri plates were associated with heterokaryon formation under selected conditions, (iv) fruit body formation was dependent upon the mating type gene, and (v) compatible mating types may not always be sufficient for fruiting.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bölker M., Urban M., Kahmann R. The a mating type locus of U. maydis specifies cell signaling components. Cell. 1992 Feb 7;68(3):441–450. doi: 10.1016/0092-8674(92)90182-c. [DOI] [PubMed] [Google Scholar]
- Castle A. J., Horgen P. A., Anderson J. B. Crosses among Homokaryons from Commercial and Wild-Collected Strains of the Mushroom Agaricus brunnescens (= A. bisporus). Appl Environ Microbiol. 1988 Jul;54(7):1643–1648. doi: 10.1128/aem.54.7.1643-1648.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle A. J., Horgen P. A., Anderson J. B. Restriction fragment length polymorphisms in the mushrooms Agaricus brunnescens and Agaricus bitorquis. Appl Environ Microbiol. 1987 Apr;53(4):816–822. doi: 10.1128/aem.53.4.816-822.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS H. J. Nuclear behaviour in the cultivated mushroom. Chromosoma. 1959;10(2):115–135. doi: 10.1007/BF00396566. [DOI] [PubMed] [Google Scholar]
- Fredrickson B. E., Yuan H. A., Miller H. Burst fractures of the fifth lumbar vertebra. A report of four cases. J Bone Joint Surg Am. 1982 Sep;64(7):1088–1094. [PubMed] [Google Scholar]
- Gillissen B., Bergemann J., Sandmann C., Schroeer B., Bölker M., Kahmann R. A two-component regulatory system for self/non-self recognition in Ustilago maydis. Cell. 1992 Feb 21;68(4):647–657. doi: 10.1016/0092-8674(92)90141-x. [DOI] [PubMed] [Google Scholar]
- Horton J. S., Raper C. A. A mushroom-inducing DNA sequence isolated from the Basidiomycete, Schizophyllum commune. Genetics. 1991 Nov;129(3):707–716. doi: 10.1093/genetics/129.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrigan R. W., Royer J. C., Baller L. M., Kohli Y., Horgen P. A., Anderson J. B. Meiotic behavior and linkage relationships in the secondarily homothallic fungus Agaricus bisporus. Genetics. 1993 Feb;133(2):225–236. doi: 10.1093/genetics/133.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander E. S., Green P., Abrahamson J., Barlow A., Daly M. J., Lincoln S. E., Newberg L. A., Newburg L. MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics. 1987 Oct;1(2):174–181. doi: 10.1016/0888-7543(87)90010-3. [DOI] [PubMed] [Google Scholar]
- Leslie J. F., Raju N. B. Recessive mutations from natural populations of Neurospora crassa that are expressed in the sexual diplophase. Genetics. 1985 Dec;111(4):759–777. doi: 10.1093/genetics/111.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May B., Royse D. J. Genetic variation and joint segregation of biochemical loci in the common meadow mushroom, Agaricus campestris. Biochem Genet. 1982 Dec;20(11-12):1165–1173. doi: 10.1007/BF00498940. [DOI] [PubMed] [Google Scholar]
- San Antonio J. P. A laboratory method to obtain fruit from cased grain spawn of the cultivated mushoom, Agaricus bisporus. Mycologia. 1971 Jan-Feb;63(1):16–21. [PubMed] [Google Scholar]
- Summerbell R. C., Castle A. J., Horgen P. A., Anderson J. B. Inheritance of restriction fragment length polymorphisms in Agaricus brunnescens. Genetics. 1989 Oct;123(2):293–300. doi: 10.1093/genetics/123.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich R. C., Specht C. A., Stankis M. M., Yang H., Giasson L., Novotny C. P. Molecular biology of mating-type determination in Schizophyllum commune. Genet Eng (N Y) 1991;13:279–306. doi: 10.1007/978-1-4615-3760-1_13. [DOI] [PubMed] [Google Scholar]
- Zolan M. E., Pukkila P. J. Inheritance of DNA methylation in Coprinus cinereus. Mol Cell Biol. 1986 Jan;6(1):195–200. doi: 10.1128/mcb.6.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]


