Abstract
Xenorhabdus bovienii wild-type strains lack a functional receptor protein (LamB) in the outer membrane and as a result are unable to adsorb coliphage lambda (λ). Introduction of plasmids encoding lamB into X. bovienii T228 results in constitutive expression of LamB in the outer membrane of this organism. LamB-expressing strains of X. bovienii adsorb lambda bacteriophage particles and can be used as hosts for lambda::Tn constructs. A Tn10-derived transposon, element 9 (J. C. Way, D. Davis, D. Morisato, D. E. Roberts, and N. Kleckner, Gene 32:369-379, 1984) was used to construct a variety of insertion mutants of X. bovienii. Mutants that had altered expression of protease, lipase, DNase, dye-binding capability, and hemolytic activity, in addition to a series of auxotrophic mutants, were isolated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Manning P. A., Edelbluth C., Herrlich P. Export without proteolytic processing of inner and outer membrane proteins encoded by F sex factor tra cistrons in Escherichia coli minicells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4837–4841. doi: 10.1073/pnas.76.10.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhurst R. J. Antibiotic activity of Xenorhabdus spp., bacteria symbiotically associated with insect pathogenic nematodes of the families Heterorhabditidae and Steinernematidae. J Gen Microbiol. 1982 Dec;128(12):3061–3065. doi: 10.1099/00221287-128-12-3061. [DOI] [PubMed] [Google Scholar]
- Akhurst R. J. Neoaplectana species: specificity of association with bacteria of the genus Xenorhabdus. Exp Parasitol. 1983 Apr;55(2):258–263. doi: 10.1016/0014-4894(83)90020-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fellay R., Krisch H. M., Prentki P., Frey J. Omegon-Km: a transposable element designed for in vivo insertional mutagenesis and cloning of genes in gram-negative bacteria. Gene. 1989;76(2):215–226. doi: 10.1016/0378-1119(89)90162-5. [DOI] [PubMed] [Google Scholar]
- Gutierrez C., Barondess J., Manoil C., Beckwith J. The use of transposon TnphoA to detect genes for cell envelope proteins subject to a common regulatory stimulus. Analysis of osmotically regulated genes in Escherichia coli. J Mol Biol. 1987 May 20;195(2):289–297. doi: 10.1016/0022-2836(87)90650-4. [DOI] [PubMed] [Google Scholar]
- Herrero M., de Lorenzo V., Timmis K. N. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol. 1990 Nov;172(11):6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig R. A. Gene tandem-mediated selection of coliphage lambda-receptive Agrobacterium, Pseudomonas, and Rhizobium strains. Proc Natl Acad Sci U S A. 1987 May;84(10):3334–3338. doi: 10.1073/pnas.84.10.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Manning P. A., Heuzenroeder M. W., Yeadon J., Leavesley D. I., Reeves P. R., Rowley D. Molecular cloning and expression in Escherichia coli K-12 of the O antigens of the Inaba and Ogawa serotypes of the Vibrio cholerae O1 lipopolysaccharides and their potential for vaccine development. Infect Immun. 1986 Aug;53(2):272–277. doi: 10.1128/iai.53.2.272-277.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poinar G. O., Jr, Thomas G. M. Significance of Achromobacter nematophilus Poinar and Thomas (Achromobacteraceae: Eubacteriales) in the development of the nematode, DD-136 (Neoaplectana sp. Steinernematidae). Parasitology. 1966 May;56(2):385–390. doi: 10.1017/s0031182000070980. [DOI] [PubMed] [Google Scholar]
- SIERRA G. A simple method for the detection of lipolytic activity of micro-organisms and some observations on the influence of the contact between cells and fatty substrates. Antonie Van Leeuwenhoek. 1957;23(1):15–22. doi: 10.1007/BF02545855. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Reversible interaction between coliphage lambda and its receptor protein. J Mol Biol. 1975 Nov 25;99(1):185–201. doi: 10.1016/s0022-2836(75)80167-7. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Sur l'existence chez Escherichia coli K 12 d'une régulation commune à la biosynthèse des récepteurs du bactériophage et au métabolisme du maltose. Ann Inst Pasteur (Paris) 1967 Nov;113(5):685–704. [PubMed] [Google Scholar]
- Selvaraj G., Iyer V. N. Suicide plasmid vehicles for insertion mutagenesis in Rhizobium meliloti and related bacteria. J Bacteriol. 1983 Dec;156(3):1292–1300. doi: 10.1128/jb.156.3.1292-1300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R., O'Connell M., Labes M., Pühler A. Plasmid vectors for the genetic analysis and manipulation of rhizobia and other gram-negative bacteria. Methods Enzymol. 1986;118:640–659. doi: 10.1016/0076-6879(86)18106-7. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
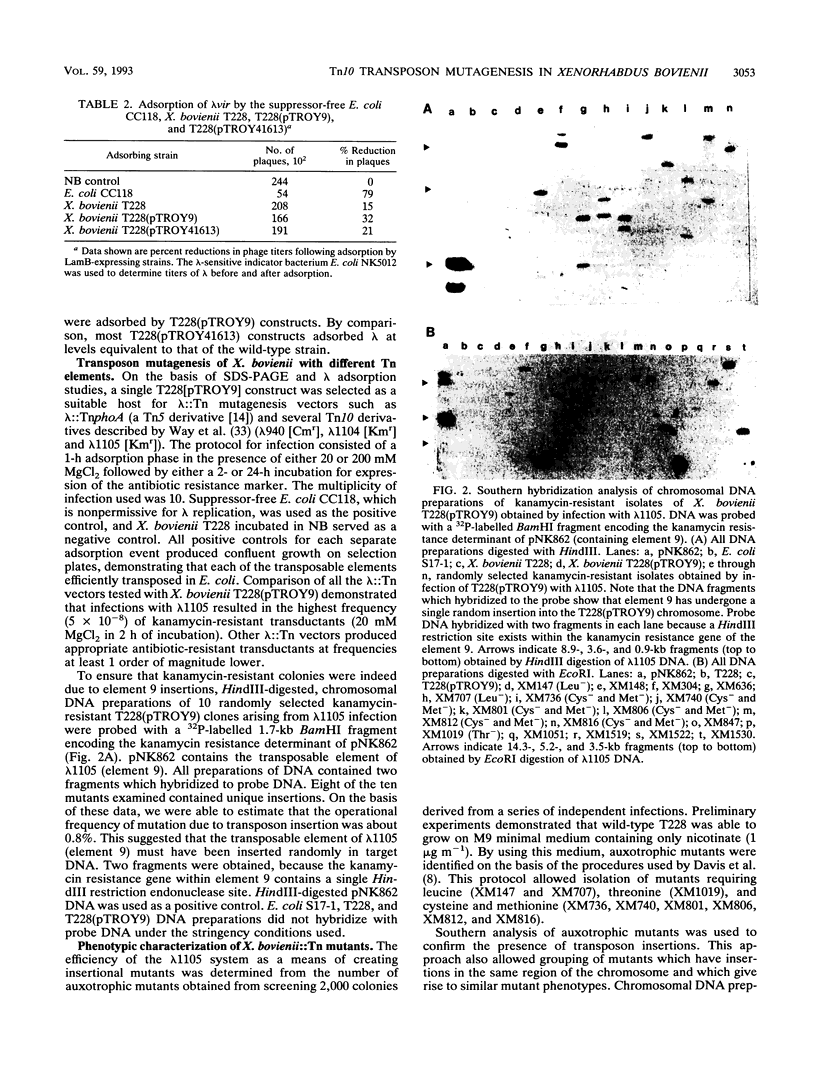
- Way J. C., Davis M. A., Morisato D., Roberts D. E., Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984 Dec;32(3):369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]
- Xu J., Olson M. E., Kahn M. L., Hurlbert R. E. Characterization of Tn5-Induced Mutants of Xenorhabdus nematophilus ATCC 19061. Appl Environ Microbiol. 1991 Apr;57(4):1173–1180. doi: 10.1128/aem.57.4.1173-1180.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn F. J., Lupski J. R. The use of transposon Tn5 mutagenesis in the rapid generation of correlated physical and genetic maps of DNA segments cloned into multicopy plasmids--a review. Gene. 1984 Feb;27(2):131–149. doi: 10.1016/0378-1119(84)90135-5. [DOI] [PubMed] [Google Scholar]
- de Vries G. E., Raymond C. K., Ludwig R. A. Extension of bacteriophage lambda host range: selection, cloning, and characterization of a constitutive lambda receptor gene. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6080–6084. doi: 10.1073/pnas.81.19.6080. [DOI] [PMC free article] [PubMed] [Google Scholar]