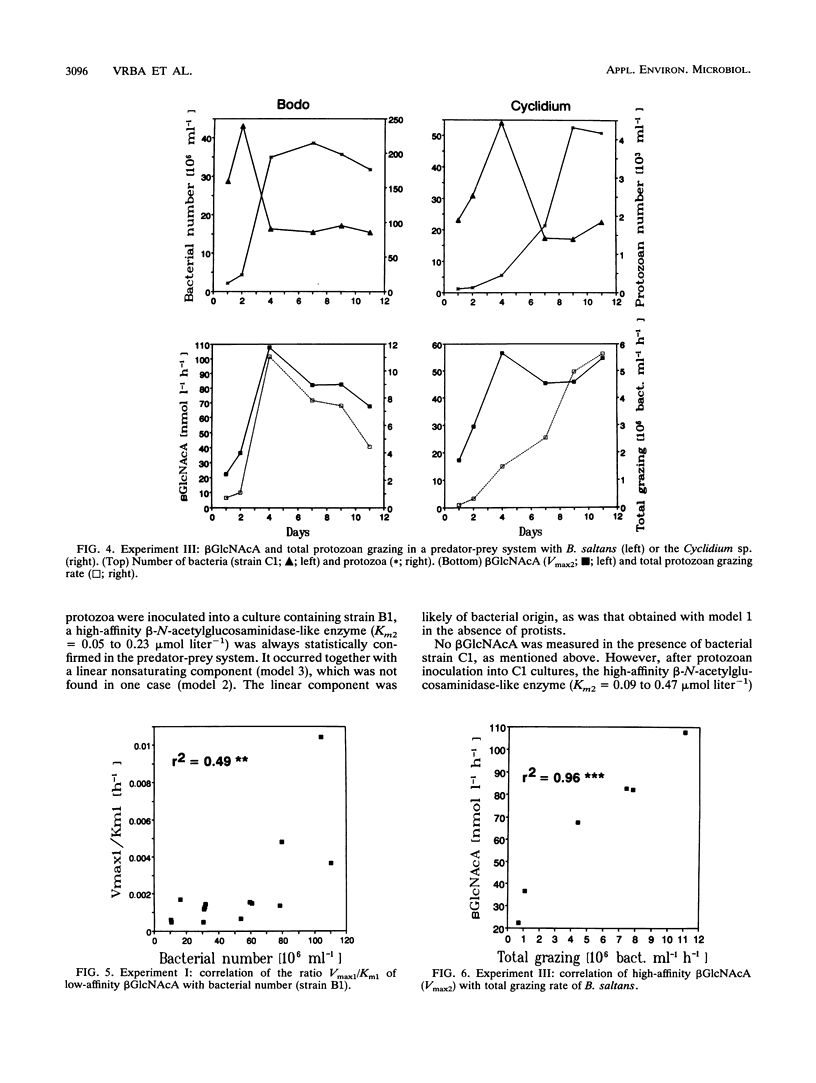
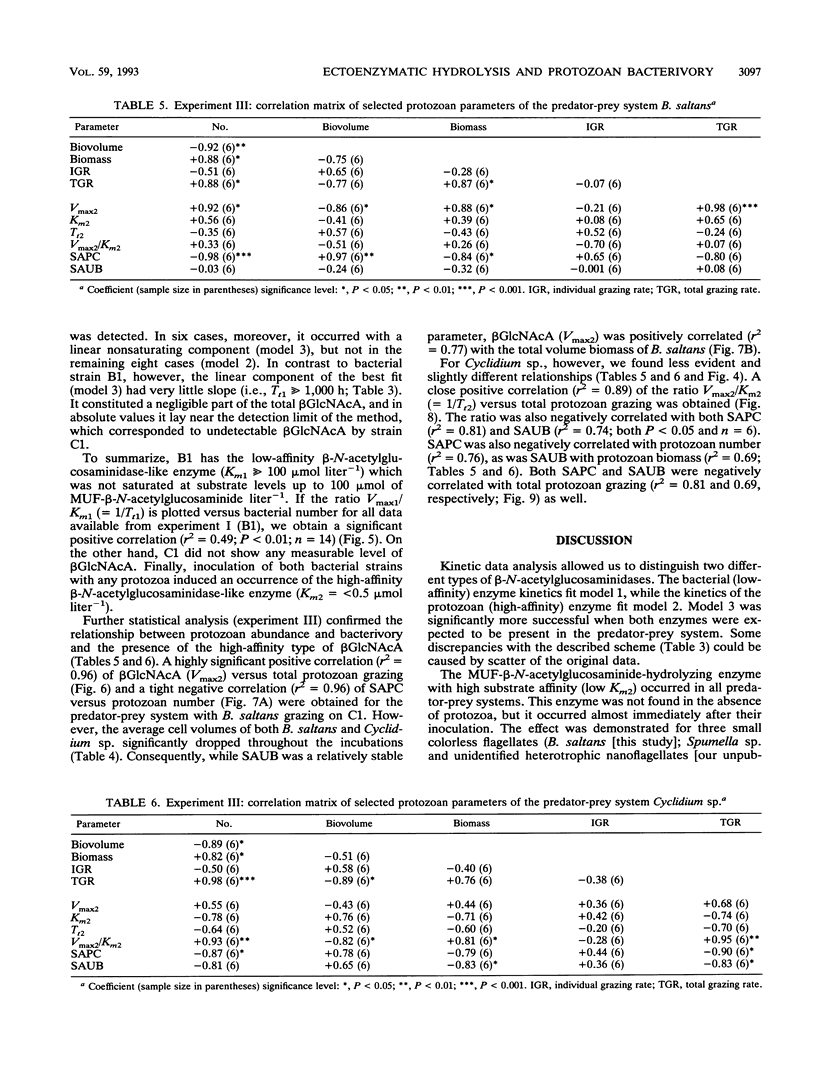
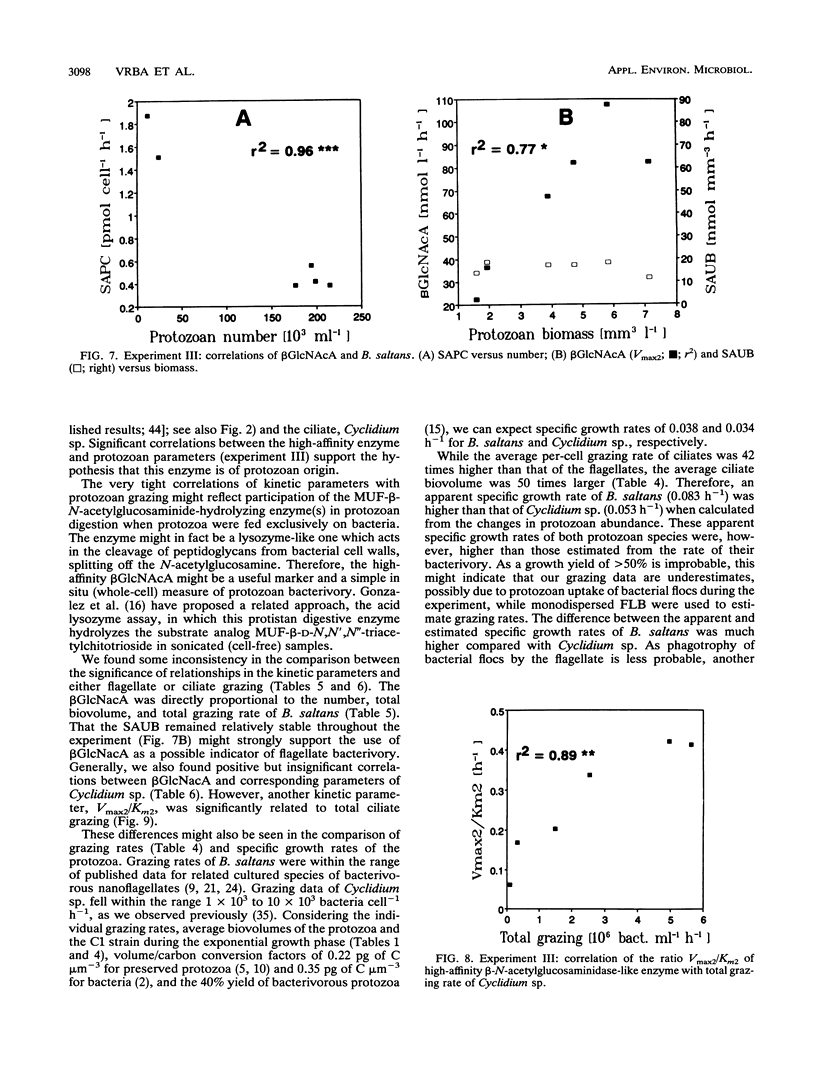
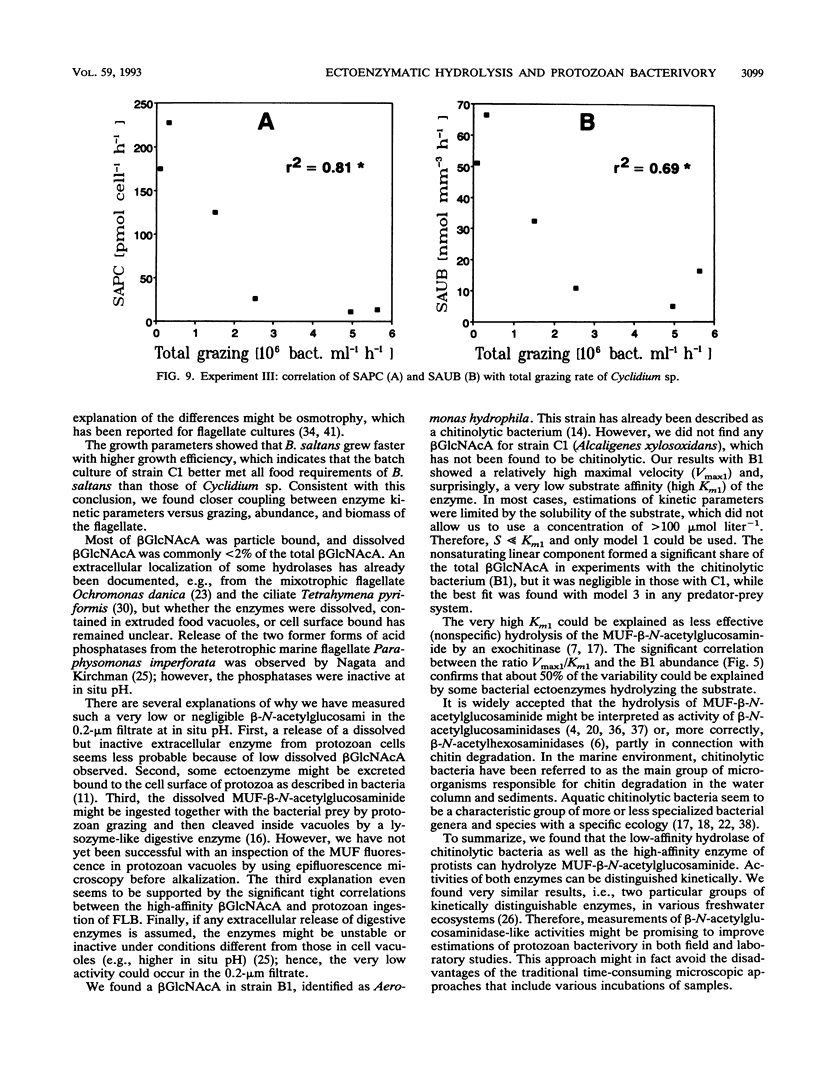
Abstract
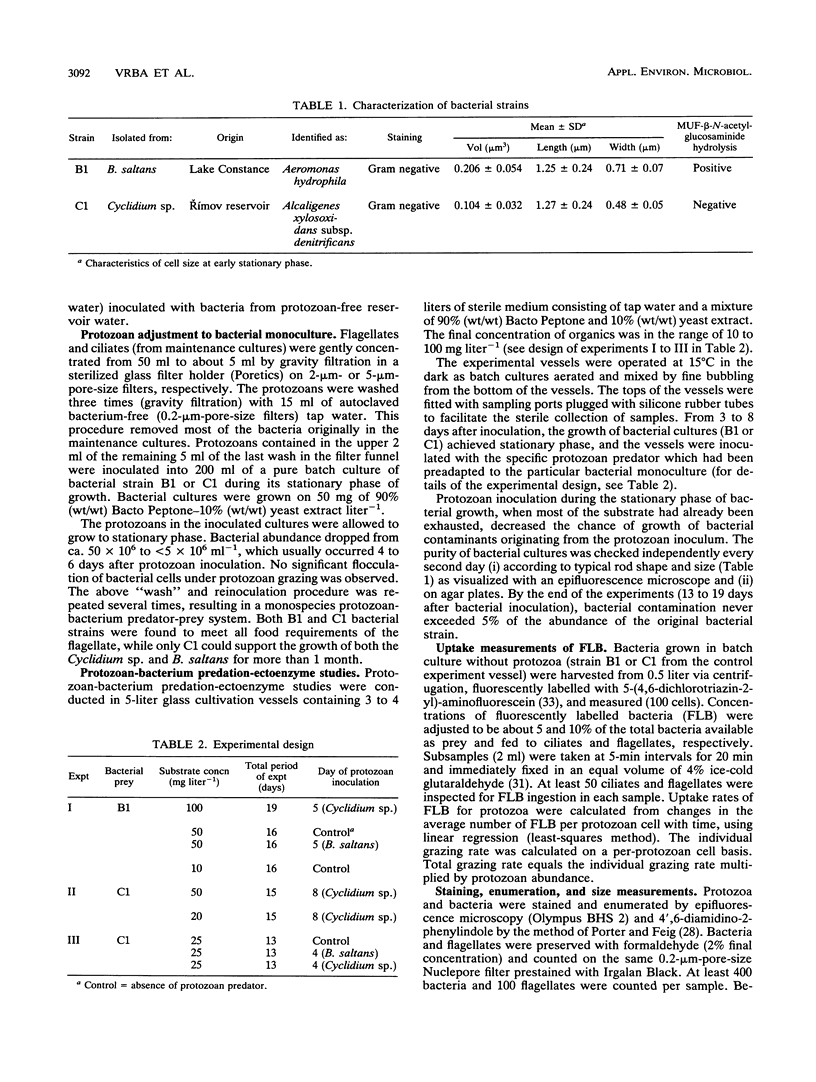
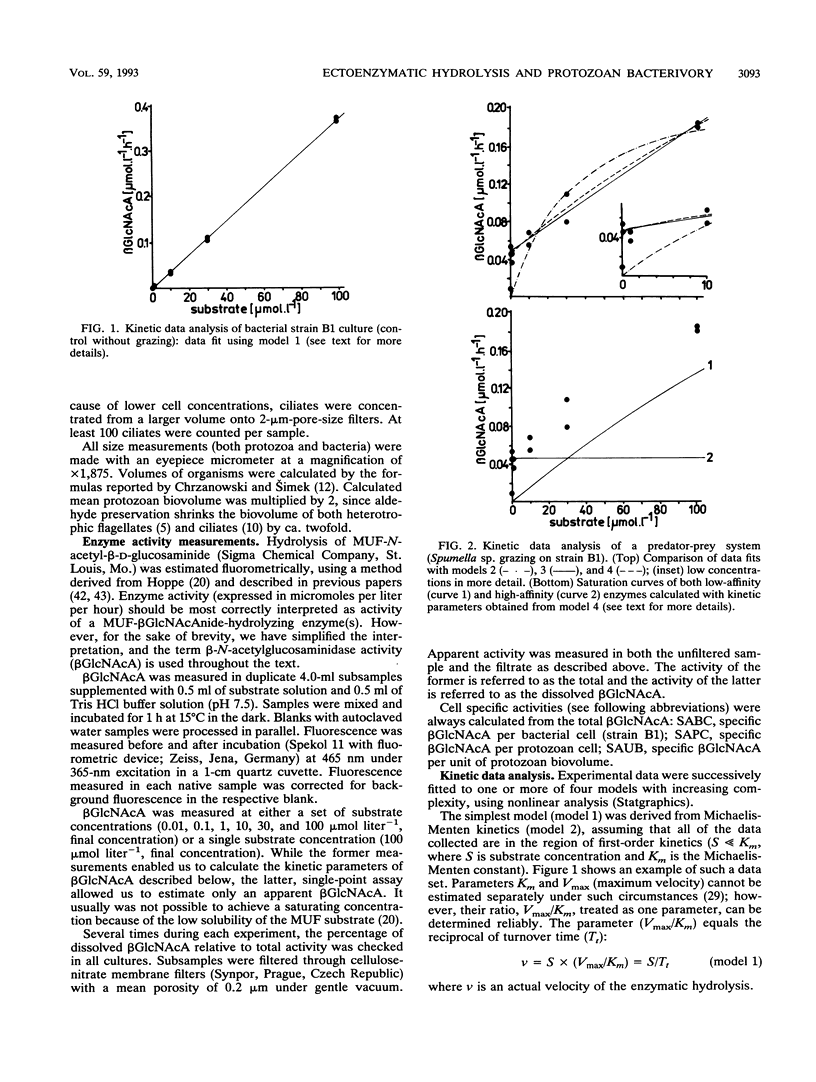
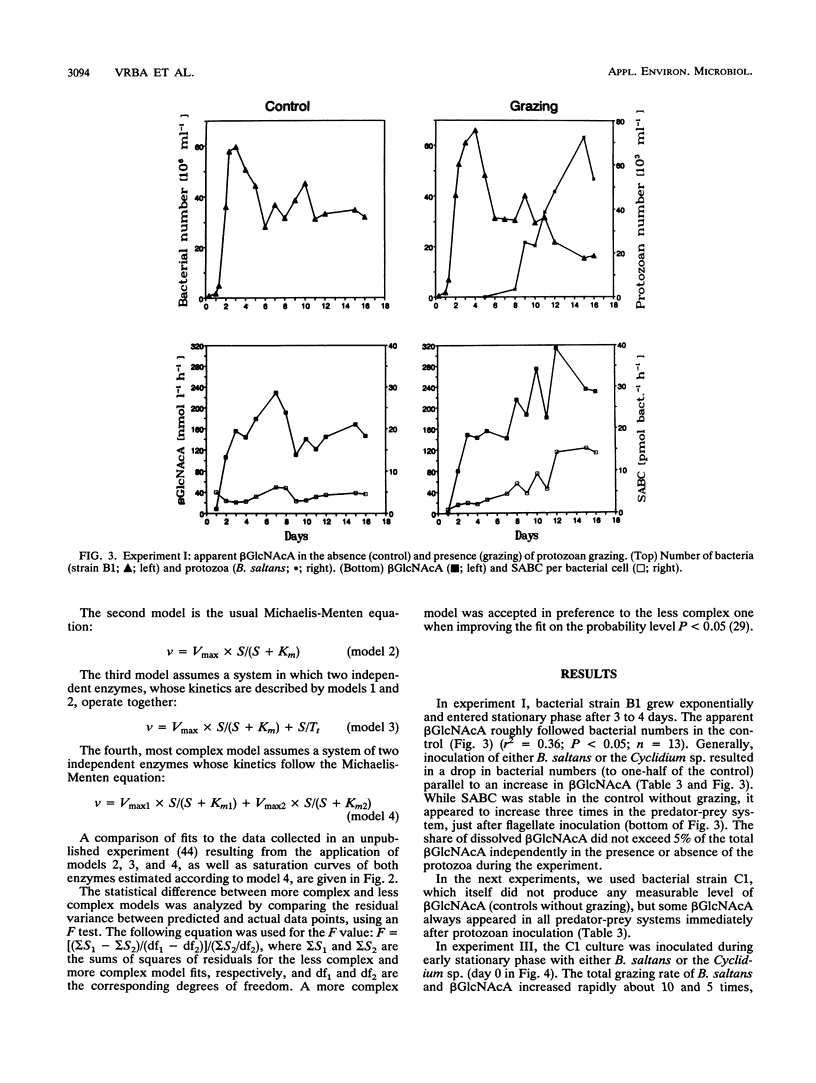
Hydrolysis of an artificial fluorogenic substrate, 4-methylumbelliferyl-β-N-acetylglucosaminide, has been studied in a monoculture predator-prey system with either a flagellate (Bodo saltans) or a ciliate (Cyclidium sp.) fed upon pure bacterial culture (Aeromonas hydrophila or Alcaligenes xylosoxidans). Aeromonas hydrophila produced a low-affinity β-N-acetylglucosaminidase-like enzyme (Km, ≫100 μmol liter-1) but Alcaligenes xylosoxidans did not. Inoculation of both bacterial strains with bacterivorous protozoa induced the occurrence of another, high-affinity, β-N-acetylglucosaminidase-like enzyme (Km, <0.5 μmol liter-1). The latter enzyme showed significant, close correlations with total grazing rates of both B. saltans (r2 = 0.96) and Cyclidium sp. (r2 = 0.89) estimated by using uptake of fluorescently labelled bacteria. Further significant correlations between several protozoan parameters and kinetic parameters of this enzyme suggest its likely protozoan origin. If both types of enzyme occurred together, they could be satisfactorily distinguished by using kinetic data analysis. Hence, measurements of β-N-acetylglucosaminidase-like activities might be promising to use to improve estimations of protozoan bacterivory.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjørnsen P. K. Automatic determination of bacterioplankton biomass by image analysis. Appl Environ Microbiol. 1986 Jun;51(6):1199–1204. doi: 10.1128/aem.51.6.1199-1204.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabezas J. A. Some comments on the type references of the official nomenclature (IUB) for beta-N-acetylglucosaminidase, beta-N-acetylhexosaminidase and beta-N-acetylgalactosaminidase. Biochem J. 1989 Aug 1;261(3):1059–1060. doi: 10.1042/bj2611059b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. W., Peters F. Effects of Temperature on Two Psychrophilic Ecotypes of a Heterotrophic Nanoflagellate, Paraphysomonas imperforata. Appl Environ Microbiol. 1992 Feb;58(2):593–599. doi: 10.1128/aem.58.2.593-599.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. W., Stoecker D. K. Effects of fixation on cell volume of marine planktonic protozoa. Appl Environ Microbiol. 1989 Jul;55(7):1761–1765. doi: 10.1128/aem.55.7.1761-1765.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D. H. Secretion of beta-glucosidase by Ochromonas danica. Arch Microbiol. 1976 Sep 1;109(3):263–270. doi: 10.1007/BF00446637. [DOI] [PubMed] [Google Scholar]
- Rothstein T. L., Blum J. J. Lysosomal physiology in Tetrahymena. II. Effect of culture age and temperature on the extracellular release of 3 acid hydrolases. J Protozool. 1974 Feb;21(1):163–168. doi: 10.1111/j.1550-7408.1974.tb03632.x. [DOI] [PubMed] [Google Scholar]
- Sherr B. F., Sherr E. B., Fallon R. D. Use of monodispersed, fluorescently labeled bacteria to estimate in situ protozoan bacterivory. Appl Environ Microbiol. 1987 May;53(5):958–965. doi: 10.1128/aem.53.5.958-965.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sochard M. R., Wilson D. F., Austin B., Colwell R. R. Bacteria associated with the surface and gut of marine copepods. Appl Environ Microbiol. 1979 Apr;37(4):750–759. doi: 10.1128/aem.37.4.750-759.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. W. Biosynthesis and composition of gram-negative bacterial extracellular and wall polysaccharides. Annu Rev Microbiol. 1985;39:243–270. doi: 10.1146/annurev.mi.39.100185.001331. [DOI] [PubMed] [Google Scholar]


