Abstract
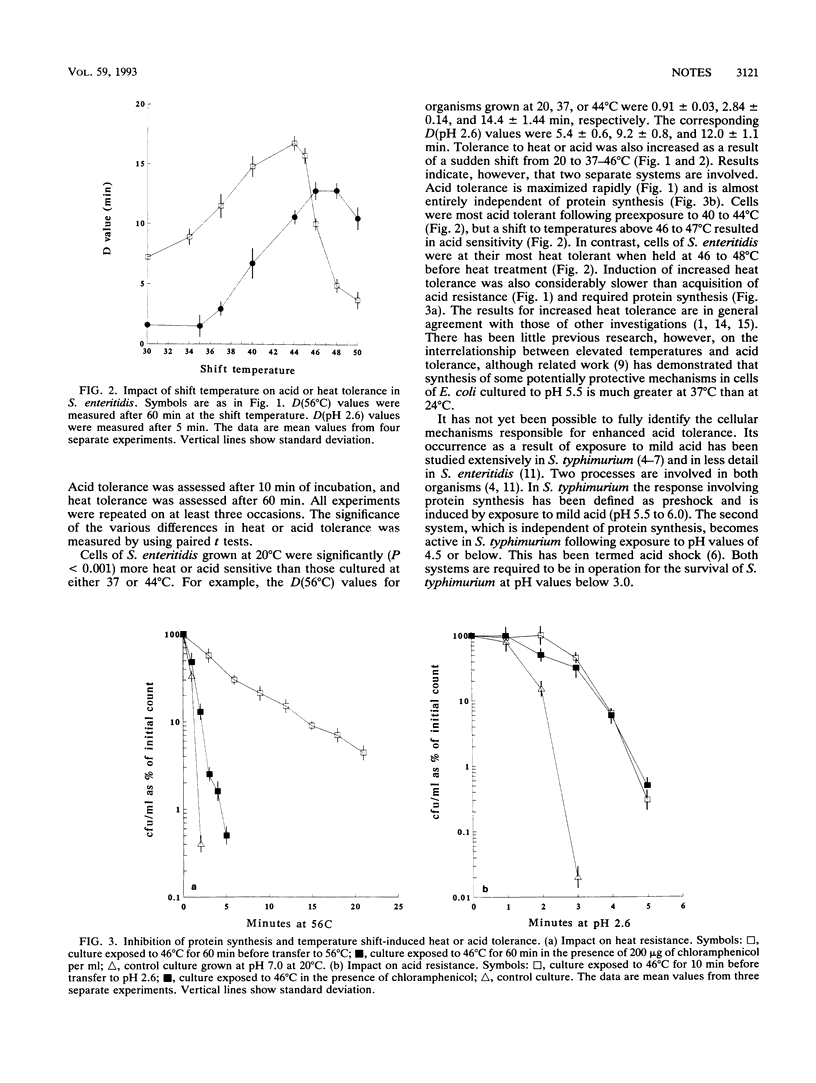
The transfer of cells of Salmonella enteritidis phage type 4 from 20 to 37-46 degrees C resulted in marked increases in acid and heat tolerance. The former was maximized within 5 to 15 min of the shift and was largely independent of protein synthesis. In contrast, induction of increased heat tolerance was slower, requiring more than 60 min to be completed, and was prevented by inhibition of protein synthesis. When cells were transferred to medium at temperatures between 47 and 50 degrees C, the kinetics of induction of heat tolerance were essentially the same as at the lower temperatures. In contrast, the cells became more acid sensitive. The results of these studies clearly show that although both acid and heat resistance can be enhanced by preexposure to high incubation temperatures, the mechanisms involved are different.
Full text
PDF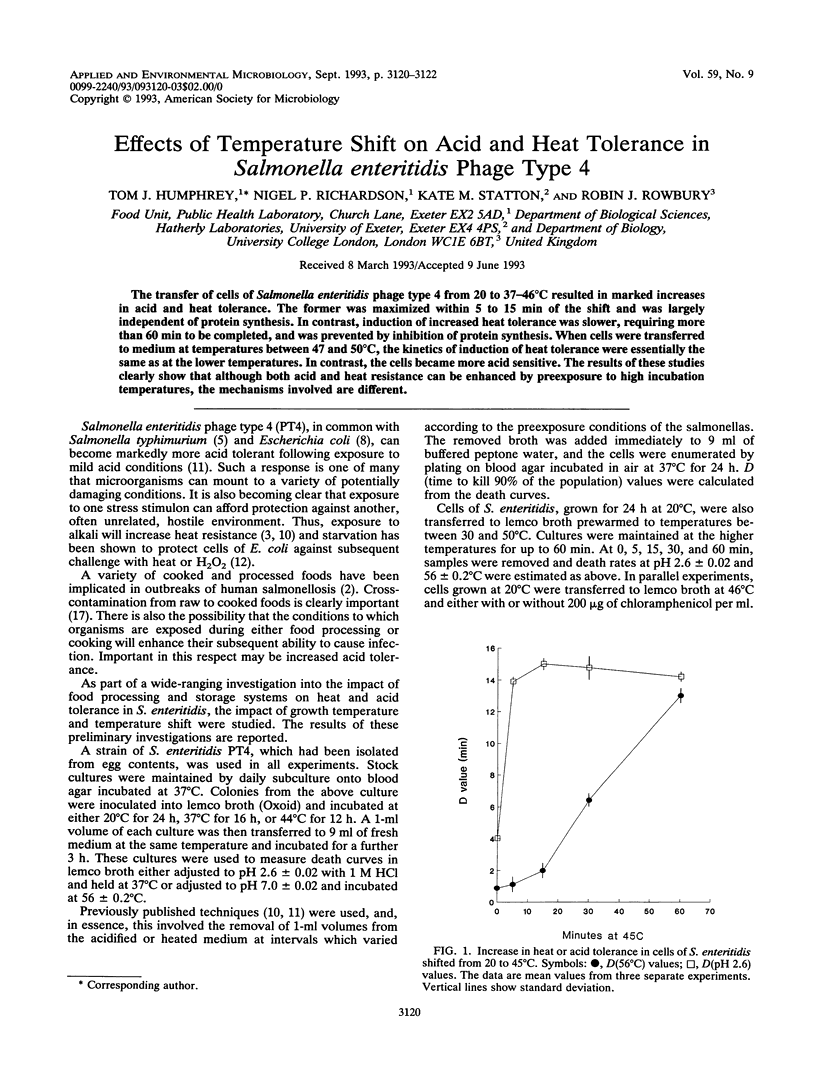

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bunning V. K., Crawford R. G., Tierney J. T., Peeler J. T. Thermotolerance of Listeria monocytogenes and Salmonella typhimurium after sublethal heat shock. Appl Environ Microbiol. 1990 Oct;56(10):3216–3219. doi: 10.1128/aem.56.10.3216-3219.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Hall H. K. Adaptive acidification tolerance response of Salmonella typhimurium. J Bacteriol. 1990 Feb;172(2):771–778. doi: 10.1128/jb.172.2.771-778.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Hall H. K. Effect of Salmonella typhimurium ferric uptake regulator (fur) mutations on iron- and pH-regulated protein synthesis. J Bacteriol. 1992 Jul;174(13):4317–4323. doi: 10.1128/jb.174.13.4317-4323.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Hall H. K. Inducible pH homeostasis and the acid tolerance response of Salmonella typhimurium. J Bacteriol. 1991 Aug;173(16):5129–5135. doi: 10.1128/jb.173.16.5129-5135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W. Salmonella acid shock proteins are required for the adaptive acid tolerance response. J Bacteriol. 1991 Nov;173(21):6896–6902. doi: 10.1128/jb.173.21.6896-6902.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani M., Pincus D. H., Bennett G. N., Hirshfield I. N. Temperature-dependent induction of an acid-inducible stimulon of Escherichia coli in broth. Appl Environ Microbiol. 1992 Aug;58(8):2704–2707. doi: 10.1128/aem.58.8.2704-2707.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins D. E., Schultz J. E., Matin A. Starvation-induced cross protection against heat or H2O2 challenge in Escherichia coli. J Bacteriol. 1988 Sep;170(9):3910–3914. doi: 10.1128/jb.170.9.3910-3914.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaux P. G., Herendeen S. L., Bloch P. L., Neidhardt F. C. Transient rates of synthesis of individual polypeptides in E. coli following temperature shifts. Cell. 1978 Mar;13(3):427–434. doi: 10.1016/0092-8674(78)90317-3. [DOI] [PubMed] [Google Scholar]
- Mackey B. M., Derrick C. M. Elevation of the heat resistance of Salmonella typhimurium by sublethal heat shock. J Appl Bacteriol. 1986 Nov;61(5):389–393. doi: 10.1111/j.1365-2672.1986.tb04301.x. [DOI] [PubMed] [Google Scholar]
- Marr A. G., Ingraham J. L. EFFECT OF TEMPERATURE ON THE COMPOSITION OF FATTY ACIDS IN ESCHERICHIA COLI. J Bacteriol. 1962 Dec;84(6):1260–1267. doi: 10.1128/jb.84.6.1260-1267.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. Factors contributing to outbreaks of food poisoning in England and Wales 1970-1979. J Hyg (Lond) 1982 Dec;89(3):491–498. doi: 10.1017/s0022172400071059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statner B., Jones M. J., George W. L. Effect of incubation temperature on growth and soluble protein profiles of motile Aeromonas strains. J Clin Microbiol. 1988 Feb;26(2):392–393. doi: 10.1128/jcm.26.2.392-393.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



