Abstract
Mutations in the potassium channel gene KCNQ4 underlie DFNA2, an autosomal dominant form of progressive hearing loss in humans. In the mouse cochlea, the transcript has been found exclusively in the outer hair cells. By using specific antibodies, we now show that KCNQ4 is situated at the basal membrane of these sensory cells. In the vestibular organs, KCNQ4 is restricted to the type I hair cells and the afferent calyx-like nerve endings ensheathing these sensory cells. Several lines of evidence suggest that KCNQ4 underlies the IK,n and gK,L currents that have been described in the outer and type I hair cells, respectively, and that are already open at resting potentials. KCNQ4 is also expressed in neurons of many, but not all, nuclei of the central auditory pathway, and is absent from most other brain regions. It is present, e.g., in the cochlear nuclei, the nuclei of the lateral lemniscus, and the inferior colliculus. This is the first ion channel shown to be specifically expressed in a sensory pathway. Moreover, the expression pattern of KCNQ4 in the mouse auditory system raises the possibility of a central component in the DFNA2 hearing loss.
The cochlea is a highly complex organ that transduces mechanical vibrations into electrical signals (1). These are then conveyed by neurons of the auditory pathway to the primary auditory cortex. As expected from the complexity of the hearing process, a large number of genes underlie human deafness. To date, 53 loci for nonsyndromic (i.e., isolated) deafness are known, and several of the corresponding genes have been identified during the past 3 years. All of these genes are expressed in the cochlea, although their expression is, in most cases, not restricted to that tissue. The encoded proteins include transcription factors, cytoskeletal components, extracellular matrix components, and transport molecules (2–4).
We have recently identified a gene encoding a potassium channel, KCNQ4, as underlying an autosomal dominant progressive deafness (DFNA2), and have shown by heterologous expression in Xenopus oocytes that a disease-causing mutation reduces potassium currents by a dominant-negative effect (5). Thereafter, four additional KCNQ4 mutations underlying DFNA2 have been reported (6). In the mouse cochlea, the orthologous transcript has been detected only in the sensory outer hair cells (OHC) (5). In contrast to the inner hair cells (IHC), which provide the main input to the auditory pathway, the major role of the OHC is the mechanical amplification and tuning of vibrations (7). OHC contract in response to the cell depolarization resulting from the opening of the mechanosensitive cation channels at the apical cell membrane. The contraction of the OHC depends on a still unidentified protein in their lateral membrane.
An important step toward elucidating the role of KCNQ4 in hearing is the determination of its cellular localization and the time of first expression in relation to the maturation of the auditory function. To this end, we generated specific antibodies to the murine KCNQ4 and studied its distribution in the inner ear and brain.
Materials and Methods
Generation of KCNQ4 Antibodies.
Immune sera against two nonoverlapping KCNQ4 synthetic peptides, corresponding to the carboxyl terminus of the protein (peptide K4C: CSISRSVSTNMD-COOH) and to the just adjacent sequence (peptide K4AC: PVDHEDISVSAQC-amide) were raised in rabbits. The sequences of these two peptides showed no homology with those of KCNQ1, KCNQ2, and KCNQ3, and reverse transcription–PCR experiments on mouse brain revealed that these sequences are identical in humans and mice. These peptides were coupled to keyhole limpet hemocyanin via a Cys residue that had been added to their amino and carboxyl terminus, respectively. After three boosts of immunization, the antisera were affinity purified. The polyclonal antibodies were tested, by immunoblotting and immunocytofluorescence, on both transfected COS-7 cells expressing KCNQ4 tagged with a myc-epitope and on nontransfected cells. Double labeling of the transfected cells with an antibody to the myc-tag revealed the colocalization of the KCNQ4 and myc fluorescence signals.
Immunofluorescence on Inner Ear Sections.
The expression of KCNQ4 was determined in C3H mice at every postnatal day (P) between P0 and P14, at P17 and P21, and in 2- to 3-month-old mice. Fixation of entire heads (P0–P14) or of dissected inner ears (from P17 onward) was performed by immersion in 4% paraformaldehyde in PBS for 4–6 h at 4°C. The samples were processed without decalcification until P5. From P6 onward, the samples were decalcified in 10% EDTA (PBS), pH 7.4, for 24 h (P6-P7), 36 h (P8-P14), or 48 h (P17 to adult), fixed again in 4% paraformaldehyde for 1 h, decalcified again in 10% EDTA for 12 h (P8-P12), 24 h (P13 and P14), or 48 h (P17-adult), rinsed twice in PBS for 10 min, and cryoprotected by immersion in 20% sucrose for 12 h. They were embedded in Tissue-Tek OCT compound (Sakura Finetek, Zoeterwoude, The Netherlands) rapidly frozen by immersion in isopentane at −60 to −70°C. Sections (10 μm) were washed three times in PBS, immersed in 0.2% glycine (PBS), washed three times in PBS, preincubated in 1.5% BSA/0.1% Triton X-100 (PBS) for 1 h, and incubated with the primary antibody (1:300) either overnight at 4°C or 2 h at room temperature. Sections were subsequently washed four times in PBS, incubated for 1 h with anti-rabbit IgG secondary antibody (Vector Laboratories) at a 1:200 dilution, washed four times in PBS, and finally covered by one drop of Vectashield mounting medium (Vector Laboratories). Microphotographs were made with a Leitz DMRB microscope. In addition, a monoclonal antibody directed against the 70- and 200-kDa neurofilament proteins (Euro-diagnostica, Arnhem, The Netherlands) was used in double-labeling experiments to detect specifically the nerve endings of type I (but not type II) hair cells in the vestibular organs.
Electronmicroscopy of Inner Ear Sections.
The postembedding Immunogold method is modified from that of Matsubara et al. (8) and has been described (9, 10). Briefly, a male Sprague–Dawley rat was perfused with 4% paraformaldehyde plus 0.5% glutaraldehyde in 0.12 M phosphate buffer. The inner ear was further perfused through the round window with the same fixative and postfixed for 2 h. The organ of Corti, crista ampullaris, and utricular macula were microdissected and washed three times for 1 h in 0.1 M PBS with 4% glucose. The samples were immersed in a series of 10, 20, and 30% glycerol and frozen in liquid propane at −184°C in a Leica EM CPC (Leica, Wetzlar, Germany). Frozen samples were immersed in 1.5% uranyl acetate in methanol at −90°C in a Leica AFS freeze-substitution instrument, infiltrated with Lowicryl HM 20 resin (Polyscience, Warrington, PA) at −45°C, and polymerized with UV light (−45 to 0°C). Ultrathin sections (75 nm) were sequentially incubated in 0.1% sodium borohydride plus 50 mM glycine in Tris-buffered saline/0.1% Triton X-100 for 10 min, 10% normal goat serum in Tris-buffered saline/0.1% Triton X-100 for 10 min, primary antibodies (mixture of anti-K4C and anti-K4AC, 1:25 dilution each) in 1% normal goat serum/Tris-buffered saline/0.1%Triton X-100 at room temperature for 2 h, anti-rabbit IgG conjugated to 10-nm gold particles (Immunogold, Goldmark Biologicals, Phillipsburg, NJ) in 1% normal goat serum/Tris-buffered saline/0.1% Triton X-100 plus 0.5% polyethylene glycol for 1 h, and finally stained in uranyl acetate and lead citrate.
Immunohistochemistry and in Situ Hybridization on Brain Sections.
Mice (strain C3H) were perfused through the left heart ventricle with 0.9% NaCl for 1 min, and 4% paraformaldehyde (PBS) for 3 min. After dissection, the brain was postfixed overnight in the same solution and immersed in 30% sucrose (PBS) for 1–2 days. Sections of 30–40-μm thickness were obtained with a vibratome (Leica). After blocking with 10% horse serum, 0.1% BSA (PBS), they were incubated overnight with the affinity-purified antibody diluted at 1:750 in 1% horse serum/0.1% BSA in PBS. After incubating with a biotinylated goat anti-rabbit IgG antibody, the sections were incubated with an avidin-peroxidase complex (Vectastain, Vector Laboratories) and finally with 0.03% diaminobenzidine/0.015% H2O2. Sections were then examined with a Zeiss Axiophot microscope. For in situ hybridization, antisense and sense (control) radioactive probes were generated from a linearized vector (pBluescript) containing a partial mouse KCNQ4 cDNA (corresponding to bp 618–1,602 of the human ORF) by using 35S-UTP and either T3 or T7 RNA polymerase. Sections were then acetylated and hybridized as described (11). They were exposed to Kodak Biomax x-ray film.
Results
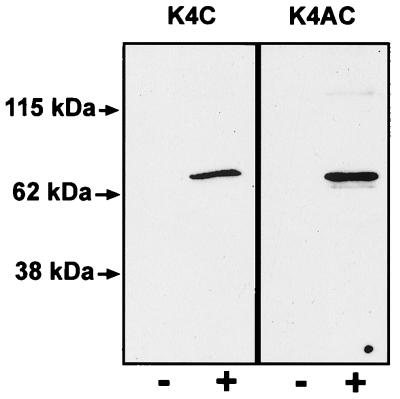
Immune sera against two nonoverlapping synthetic peptides, K4C and K4AC, were generated (see Materials and Methods). After affinity purification, the polyclonal antibodies were tested on COS-7 cells transiently transfected with KCNQ4. Both antibodies detected the protein by immunofluorescence (not shown), and recognized a single band of the expected size (≈77 kDa) on Western blot (Fig. 1). Anti-K4C gave the strongest signal and exhibited least cross-reactivity in the immunoblot analysis. In vivo, however, identical structures were labeled with either antibody, thus providing strong evidence for the specificity of the staining.
Figure 1.
Characterization of antibodies to KCNQ4. Western blot analysis of cell lysates of COS-7 cells transfected with KCNQ4 (+) and of nontransfected cells (−) as a control. The anti-K4C antibody (Left) recognized only one band in the predicted size of about 77 kDa in transfected COS-7 cell lysate. The anti-K4AC antibody recognized the same band, but on longer exposures a smaller band which was also present in nontransfected COS-7 cells became visible.
KCNQ4 in the Inner Ear.
The mammalian inner ear comprises several sensory organs: the organ of Corti in the cochlea, which is the auditory component, and five vestibular organs, which are responsible for the control of balance. These consist of the saccular and utricular maculae, both detecting linear acceleration, and the ampullar cristae of the three semicircular canals, sensitive to angular acceleration.
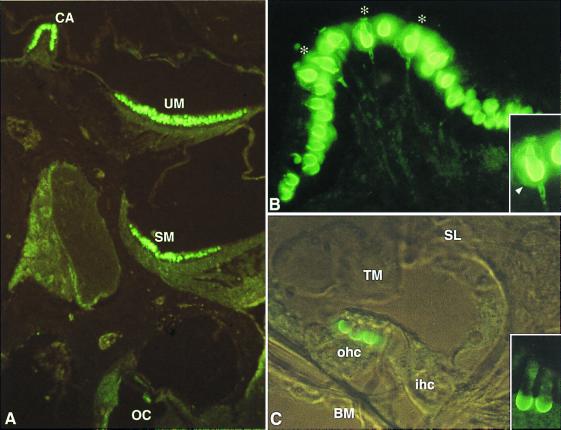
Consistent with results from in situ hybridization (5), OHC are the only cells of the cochlea that were labeled with the antibodies to KCNQ4 on cryosections (Fig. 2 A and C). No immunoreactivity was detected in the IHC or the supporting cells of the organ of Corti, nor was any labeling present in the other cochlear structures including the stria vascularis. In OHC, KCNQ4 was detected exclusively in the basal membrane, and not in the apical or lateral membrane (Fig. 2C). Immunoreactivity was first detected at P8 at the basal turn of the cochlea. In accordance with the well established basis toward apex maturation of the organ of Corti (12), the labeling then proceeded along the cochlear axis to reach the apical OHC at P13–P14. However, even in adult animals, the immunoreactivity of the most apical OHC remained weaker than that of the more basal cells. Immunoelectron microscopy confirmed that KCNQ4 is situated at the OHC basal membrane (not shown).
Figure 2.
KCNQ4 in the murine inner ear. (A) Immunofluorescence of an inner ear section at P21. KCNQ4 immunoreactivity is present in hair cells of the crista ampullaris (CA), utricular macula (UM), and saccular macula (SM), as well as in the OHC of the organ of Corti (OC). (B) Section of a crista ampullaris at P21. Cells are labeled at their basal and lateral membrane. Some of these cells (indicated by asterisks) can be unambiguously identified as type I hair cells because of the costaining of their characteristic ensheathing nerve calyx (arrowhead in Inset). Note that the staining of the calyx extends to the adjacent region of the nerve fiber. (C) Transverse section of the organ of Corti at P13 (transmitted light interference contrast). The three OHC (ohc) are labeled at their basal membrane, whereas the IHC (ihc) is not. A detailed view of two OHC is presented in the inset. BM, basilar membrane; SL, spiral limbus; and TM, tectorial membrane.
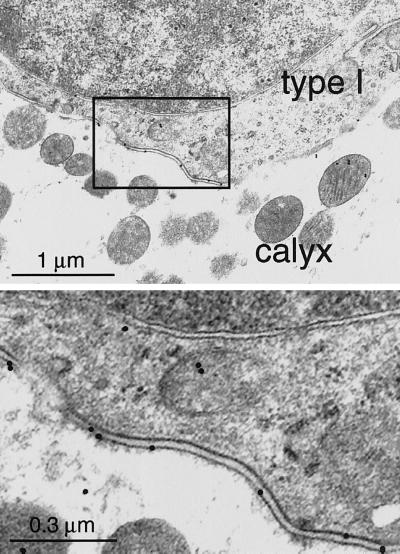
KCNQ4 was also detected in vestibular hair cells, at their basal and lateral membrane (Fig. 2 A and B). Their staining was even more intense than the one of cochlear OHC. Labeling was observed from P1-P2 onward in the ampullar cristae, and from P3 onward in the utricular and saccular maculae. Whereas initially only a few labeled cells in the central region of these structures were detected, immunoreactive cells were distributed over the entire sensory epithelia several days later. However, even in adult animals, KCNQ4 staining was restricted to a subpopulation of hair cells. Some immunoreactive cells could be at once identified as type I hair cells because their single afferent, calyx-like nerve endings were stained as well (see Fig. 2B). Furthermore, double-labeling experiments with a monoclonal antibody staining neurofilaments exclusively in the nerve endings of type I cells (13) revealed that all KCNQ4-expressing cells were of type I (data not shown). The absence of KCNQ4 labeling of type II hair cells (which lack the calyx nerve ending) was confirmed by immunoelectron microscopy. Ultrastructural analysis of type I hair cells and their ensheathing calyces revealed that KCNQ4 immunoreactivity was present in both presynaptic (hair cell) and postsynaptic (calyx of nerve ending) membranes (Fig. 3). Of a total of 113 membrane-associated gold particles, 37 were located on the presynaptic (type I cell) and 76 on the postsynaptic (calyx) membranes, respectively. Although the nerve calyx extends nearly up to the apex of the type I hair cell (14, 15), labeling of the hair cell and calyx membranes was concentrated in the basal half of this structure (Fig. 2B). Finally, KCNQ4 immunoreactivity could not be detected in the vestibular and cochlear ganglia.
Figure 3.
Ultrastructural localization of KCNQ4 in the ampullar crista (adult rat). The basal part of the synaptic cleft between a type I hair cell and the afferent nerve ending (calyx) is shown. Consistent with the light microscopy analysis (see Fig. 2B), most of the gold particles were observed in the area corresponding to the basal and basolateral parts of the type I cell membrane. (Lower) A higher magnification of the area outlined in Upper.
Distribution of KCNQ4 in the Brain.
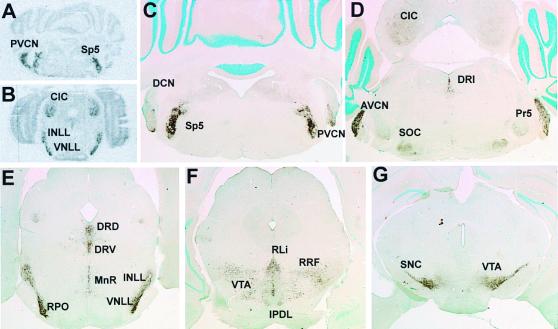
Because previous reverse transcription–PCR experiments and Northern blot analysis had indicated that KCNQ4 is also weakly expressed in the whole brain (5), we performed in situ hybridization and immunohistochemistry on mouse brain sections. We found that the mRNA and protein were expressed exclusively in structures of the brainstem. Interestingly, most labeled nuclei belonged to the auditory pathway (Fig. 4). A prominent staining was found in the anterior and posterior ventral cochlear nuclei, but not in the dorsal cochlear nucleus (Fig. 4 A, C, and D). Because the protein was concentrated at somata and dendrites, the labeled neurons could be identified as being multipolar (Fig. 5 A and B). The superior olivary complex receives input from the anterior ventral cochlear nucleus and relays it to ipsi- and contralateral nuclei in the lemniscus lateralis and colliculus inferior. The superior olivary complex displayed a diffuse, moderate staining of the neuropil (Fig. 5C) in the lateral superior olive, the medial superior olivary nucleus, but not the medial nucleus of the trapezoid body (Fig. 4D). The cochlear nucleus and superior olivary complex project to the lateral lemniscus where KCNQ4 was strongly expressed in its dorsal, intermediate, and ventral nuclei (Fig. 4E). The next labeled structure of the auditory pathway was the central nucleus of the inferior colliculus (Fig. 4 D and E). It displayed a moderate KCNQ4 expression of its neuropil, similar to that of the superior olivary complex (Fig. 5D). Other nuclei of the auditory pathway as the medial geniculate body and the primary auditory cortex neither showed mRNA expression nor obvious protein levels. Additionally, cochlear root neurons were immunoreactive (Fig. 5E). These neurons, which are involved in a fast startle response, are located in the auditory nerve before it enters the brainstem and directly receive input from many neurons which encode broad ranges of frequencies (16, 17). Although the main nuclei of the auditory pathway expressed KCNQ4, the cochlear nerve and tracts were not stained. However, fibers of the vestibular nerve (Fig. 5E) and their terminals in the vestibular nuclei were labeled. This agrees with the labeling of dendrites innervating vestibular, but not cochlear hair cells.
Figure 4.
KCNQ4 in the adult mouse brainstem. (A and B) In situ hybridization with a KCNQ4 antisense probe. (C–G) Immunohistochemistry on transverse sections of the brainstem at different levels (from caudal to rostral). The distribution of the protein is consistent with the results of in situ hybridization. KCNQ4 immunoreactivity is detected in several nuclei of the auditory pathway, namely anteroventral and posteroventral cochlear nuclei (AVCN and PVCN, respectively), superior olivary complex (SOC), ventral and intermediate nuclei of lateral lemniscus, rostral periolivary region (RPO) and the central nucleus of inferior colliculus (CIC), as well as some other nuclei, namely dorsal, ventral, and inferior nuclei of dorsal raphe (DRD, DRV, and DRI), rostral nucleus of linear raphe (RLi), nucleus of median raphe (MnR), principal and spinal trigeminal nuclei (Pr5 and Sp5), dorsal subnucleus of interpeduncular nucleus (IPDL), ventral tegmental area (VTA), retrorubral field (RRF), and pars compacta of substantia nigra (SNC).
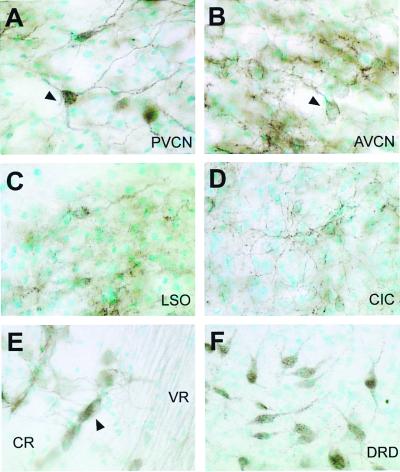
Figure 5.
Neuronal distribution of KCNQ4 in several brainstem nuclei. Labeling of several brainstem regions with the KCNQ4 antibody at higher magnification. Multipolar immunoreactive neurons (arrowheads) can be recognized in posteroventral (PVCN) (A) and anteroventral (AVCN) (B) cochlear nuclei. (C and D) Neurons from the lateral superior olive (LSO) and the central nucleus of inferior colliculus (CIC), respectively, with relatively poorly labeled somata but intensely stained neuropil. (E) A section from the eighth nerve just before its entry into the brainstem. Both cochlear root neurons (arrowhead) that are located between axons of the cochlear root (CR) (which is not labeled), and axons of the vestibular root (VR) are labeled. (F) Neurons from the dorsal nucleus of the dorsal raphe (DRD) with intense staining of somata and dendrites.
Some other brainstem regions were also positive with KCNQ4 antibodies. A strong signal was found in the two sensory nuclei (spinal Sp5 and principal Pr5) of the trigeminal nerve (Fig. 4 C and D). Many raphe nuclei were heavily stained (Fig. 4 D–F). This staining was much stronger in cell bodies and dendrites (Fig. 5F). Prominent KCNQ4 labeling was also obtained in some dopamine-rich structures as the substantia nigra pars compacta, cells of the ventral tegmental area and of the retrorubral field (Fig. 4 F and G). The pattern of staining was similar to that of the raphe nuclei. Only moderate KCNQ4 expression was observed in the dorsolateral interpeduncular nucleus and locus coeruleus.
We compared the expression of KCNQ4 during the postnatal development in mice at days P0, P5, and P10. The brain of newborn animals showed no significant expression of KCNQ4. Animals at P5 already showed low expression of KCNQ4 in the lemniscus lateralis, anterior and posterior ventral cochlear nuclei, and the raphe dorsalis (data not shown). At P10, KCNQ4 was observed additionally in the spinal trigeminal nucleus, the nucleus raphe dorsalis, and the pars compacta of the substantia nigra. Areas with more moderate staining in the adult brain had no detectable KCNQ4 expression at these earlier stages.
Discussion
To gain insights into the pathophysiology of the progressive human deafness that is caused by mutations in KCNQ4, we have analyzed the expression of this potassium channel in the murine inner ear and brain. In the ear, it is detected in OHC (but not IHC) of the organ of Corti, and in type I (but not type II) vestibular hair cells. Interestingly, it is also expressed in the postsynaptic membrane of the calyx nerve endings innervating type I cells. Most surprisingly, whereas being absent from most brain regions, KCNQ4 is expressed in several nuclei of the central auditory pathway in the brainstem.
The present study reveals that the KCNQ4 potassium channel is present only in the basal membrane of OHC. This precludes a function as either mechanosensitive channel or motor protein, because these must be localized to the apical or lateral membrane, respectively. This basal localization is consistent with the idea (5) that KCNQ4 might influence the electrical properties of OHC, or may provide an exit pathway for K+ which is taken up by the supporting cells that face the basal membrane of OHC. This localization also agrees with the distribution of K+ conductance observed along the axis of isolated OHC (18).
Also in the vestibular organ, KCNQ4 is expressed in a subset of hair cells, the type I cells. Nearly the entire basolateral membrane of these cells is ensheathed by the dendritic calyx of the afferent neuron, with which it makes 10–20 synapses that are concentrated in the basal third of the hair cell (14). KCNQ4 expression is concentrated in this basal area, and electron microscopy revealed its presence both pre- and postsynaptically. The unusual long, narrow cleft between the calyx and the hair cell is expected to drastically influence synaptic transmission. Model calculations predict a significant K+ accumulation in these clefts during hair cell excitation (19). This particular situation might “solve” the problem of how transmitter release would occur from type I cells, given their relatively negative resting potentials and small receptor potentials “in vitro ” (20). Raising the K+ concentration in these clefts will depolarize the basal membrane of the hair cell, eventually leading to an enhanced fusion of synaptic vesicles. This depolarization would not be observed in the usual whole-cell recordings, because the calyx ending is not intact. Furthermore, if the postsynaptic membrane of the nerve calyx expresses K+ channels, K+ may directly depolarize the dendritic ending (19). This is supported by the present Immunogold analysis which shows that KCNQ4 is expressed postsynaptically. This raises the possibility that K+ could serve as a “cotransmitter” in this specialized synapse. Furthermore, and similar to cochlear hair cells, also vestibular hair cells need to extrude the potassium they have taken up via the apical mechanosensitive channel. If the potassium concentration in the cleft gets as high as indicated by the model calculations (19), it might be taken up by the (KCNQ4-containing) K+ channels in the postsynaptic membrane.
It is instructive to compare the expression patterns and electrophysiological properties of KCNQ4 with native currents of hair cells. The input conductance (mediated mainly by K+ currents) is higher in type I vestibular cells than in OHC (21–23). This correlates roughly with the expression levels of KCNQ4. OHC express at least two distinct outwardly rectifying K+ currents named IK,n and IK (18, 23, 24), both of which, however, seem to activate faster than KCNQ4 (5). The voltage dependence of KCNQ4 [V1/2 ≈ −10 mV (ref. 5)] agrees better with IK (V1/2 ≈ −25 mV) than with IK,n (V1/2 between −70 and −100 mV), but IK is sensitive to 100 μM 4-aminopyridine (22, 23), which does not significantly inhibit KCNQ4 (5).
Several findings suggest that KCNQ4 mediates the IK,n current of OHC. In the mouse, this current is absent at P0 and is first seen at P8 (25), which coincides with the onset of motility of the OHC. This agrees fairly well with the temporal expression pattern of KCNQ4 determined here. In the guinea pig, there is a gradient of IK,n current from the basal to the apical turns of the cochlea (23). KCNQ4 immunoreactivity was weaker in the most apical OHC of the mouse. In the guinea pig, which has 4 cochlear coils instead of the 1.75 coils in the mouse, a conspicuous difference in the intensity of the KCNQ4 immunoreactivity was observed, with only weak OHC labeling in the most apical coil (data not shown). Furthermore, and again similar to KCNQ4, IK,n currents are concentrated at the basal pole of isolated OHC (18). Finally, recent experiments have shown that IK,n, and no other current of OHC or IHC, is inhibited by linopirdine (25). This is a rather specific inhibitor of KCNQ channels, including KCNQ4 (5, 26).
A similar situation exists for the vestibular end organs. Both types I and II hair cells express a delayed rectifier current (gDR) that activates at voltages positive to −55 mV. Type I cells have an additional outward rectifier (gK,L) which activates at more negative voltages (20, 21). Although the voltage dependence of gDR agrees better with that of KCNQ4, the presence in both type I and II cells and its presence in neonatal hair cells (21) excludes the possibility that it is mediated by KCNQ4. In contrast, gK,L is first detected at the same time as is KCNQ4 (21) and is present only in type I cells. It remains to be shown whether gK,L is sensitive to linopirdine.
Thus, KCNQ4 probably contributes to currents that are active already at the resting potential and that were called IK,n and gK,L in OHC and type I hair cells, respectively. Such a voltage dependence is compatible with the proposed roles in K+ efflux and the synaptic transmission at the specialized calyx-like synapses in the vestibular organs. However, when expressed in Xenopus oocytes, KCNQ4 activates more slowly and at more depolarized voltages than these native currents (5). Some of these differences may be caused by the experimental procedures. For instance, the enzymatic digestion used to isolate vestibular hair cells shifted the activation curve of gK,L and eliminated several currents (27, 28). Currents may be affected by the internal dialysis of cells during whole-cell recordings (28). In addition, posttranslational modifications or an association with other subunits may modify KCNQ4 currents. For instance, KCNQ4 can form heteromeric channels with KCNQ3 (5). It is presently unclear whether this occurs in hair cells and whether other KCNQ or KCNE (29) proteins interact with KCNQ4 in the ear.
One of the most intriguing findings is the expression of KCNQ4 in brain. In stark contrast to KCNQ2 and KCNQ3, which are broadly expressed in many regions of the central nervous system (30, 31), KCNQ4 expression is limited to certain brainstem nuclei. These are mainly, but not exclusively, nuclei and tracts of the auditory pathway. KCNQ4 is absent from more central structures of this pathway (like the medial geniculate body and the auditory cortex) and is not expressed in all brainstem nuclei involved in auditory processing. Its expression seems limited to neurons, where, depending on the particular area or cell type, it is expressed mainly on cell bodies or dendrites. This suggests that it is predominantly located postsynaptically, as is the case for the calyx endings on type I vestibular hair cells. However, a presynaptic localization in some neurons cannot be excluded.
Auditory processing by brainstem neurons includes sound localization, middle ear and startle reflexes, and the integration of other sensory inputs. For instance, somatosensory neurons from the spinal trigeminal nucleus project to the cochlear nucleus and the inferior colliculus (32). Interestingly, neurons of the spinal trigeminal nucleus are intensely stained for KCNQ4 (Fig. 4C). Not all neurons expressing KCNQ4 are involved in processing auditory information, but it is interesting that also the ventral tegmental area receives significant input from the cochlear nucleus (33), that some cells in the substantia nigra are connected to the inferior colliculus (34, 35) as are cells from several nuclei of the raphe and the locus coeruleus (36, 37). Neurons in these regions are also labeled for KCNQ4 (Fig. 4).
The surprisingly specific expression of KCNQ4 in the auditory pathway has no parallel in any other known neuronal systems. It is unclear whether there is any functional reason for this specificity. The auditory pathway may be unique in its fast synaptic transmission and its need for precise timing (38, 39). This seems to require substantial expression levels of outwardly rectifying K+ currents which ensure short membrane time constants, resulting in short excitatory postsynaptic potentials and action potentials (38, 39). In some nuclei, e.g., in the medial nucleus of the trapezoid body which lacks KCNQ4 expression, these currents were tentatively ascribed to distinct Kv K+ channels (40, 41). In addition to their elevated expression in some auditory nuclei, these channels are broadly expressed in several brain regions (40, 41). KCNQ4, which is much more specific for auditory structures, may serve a similar role. Like KCNQ2 and KCNQ3, which probably form one type of M current (26), KCNQ4 may also modulate neuronal excitability. It cannot be argued that the expression pattern of KCNQ4 results from a common ontogenetic origin of the respective cells, because brainstem neurons are derived from the neuroectoderm and sensory hair cells from the otic placode, that is, nonneural ectoderm (42).
Assuming similar patterns of KCNQ4 expression in mice and in humans, this work has important implications for the pathophysiology of DFNA2. Because KCNQ4 is probably mediating the IK,n current which is quantitatively important in OHC and is already open at resting potentials, expression of KCNQ4 will influence the electric properties of OHC. However, DFNA2 patients apparently hear normally during early life (5, 43). This indicates that a normal amount of KCNQ4 is not essential for OHC signal transduction, i.e., that the currents that remain with dominant negative mutations (≈10% of wild type in Xenopus oocytes; ref. 5) are sufficient for this function. However, a total elimination might lead to direct effects on signal transduction. The slowly progressive hearing loss can most easily be attributed to a degenerative process of OHC which may result from a chronic depolarization or potassium overload. Second, although KCNQ4 is expressed in the vestibular system, DFNA2 patients apparently have no balance problems (5, 43). This is not unusual because humans can adapt well to vestibular disturbances (44). More important is the possibility, which is raised by the expression in the central auditory pathway, that the deafness in DFNA2 has a central component. Indeed, because a selective loss of OHC induced by aminoglycoside injection results only in a 30–50 dB hearing loss in the chinchilla (45), the disruption of OHC function on its own may not account for the severe to profound deafness observed at the end stage of DFNA2. It will be interesting to investigate by auditory brainstem response recordings whether young patients with DFNA2, who have not yet progressed to a severe stage, show signs of an involvement of the auditory pathway. Unfortunately, the members of the family we have studied (5) had already lost too much of their hearing ability to allow such studies. Finally, one should consider the possibility that the putative primary OHC defect in DFNA2 eventually also affects the function of IHC. To determine the roles of degenerative processes and of an involvement of the central auditory pathway in DFNA2 will require the generation and analysis of an appropriate mouse model.
Acknowledgments
We thank Robert Wenthold for his support, Ronald Petralia and Ya-Shan Wang for technical help in electron microscopy, Susanne Fehr for the in situ hybridization, Christian Kubisch, Björn C. Schroeder, and Eckhard Friauf for useful discussions, and Jacqueline Levilliers for assistance in the preparation of the manuscript. This work was supported by grants from the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie to T.J.J., and by the European Economic Community (BMH4-CT96) to C.P.
Abbreviations
- OHC
outer hair cells
- IHC
inner hair cells
- DFNA
autosomal dominant deafness
- P
postnatal day
Note Added in Proof
Recent whole-mount KCNQ4 immunofluorescence experiments in the mouse and guinea pig revealed a membrane labeling of the IHC in the apical half of the cochlea. This labeling has not been detected in cryostat sections. The signal ratio of the IHC versus OHC was higher in the guinea pig. In addition, we observed a conspicuous staining of neuronal bodies in the cochlear ganglion of the guinea pig (but not of the mouse). Therefore, the possibility of an interspecies variation of KCNQ4 expression levels should be considered when discussing the pathophysiology of DFNA2.
Footnotes
See commentary on page 3786.
References
- 1.Nobili R, Mammano F, Ashmore J. Trends Neurosci. 1998;21:159–167. doi: 10.1016/s0166-2236(97)01192-2. [DOI] [PubMed] [Google Scholar]
- 2.Petit C. Nat Genet. 1996;14:385–391. doi: 10.1038/ng1296-385. [DOI] [PubMed] [Google Scholar]
- 3.Kalatzis V, Petit C. Hum Mol Genet. 1998;7:1589–1597. doi: 10.1093/hmg/7.10.1589. [DOI] [PubMed] [Google Scholar]
- 4.Holme R H, Steel K P. Curr Opin Genet Dev. 1999;9:309–314. doi: 10.1016/s0959-437x(99)80046-x. [DOI] [PubMed] [Google Scholar]
- 5.Kubisch C, Schroeder B C, Friedrich T, Lütjohann B, El-Amraoui A, Marlin S, Petit C, Jentsch T J. Cell. 1999;96:437–446. doi: 10.1016/s0092-8674(00)80556-5. [DOI] [PubMed] [Google Scholar]
- 6.Coucke P J, Hauwe P V, Kelley P M, Kunst H, Schatteman I, Velzen D V, Meyers J, Ensink R J, Verstreken M, Declau F, et al. Hum Mol Genet. 1999;8:1321–1328. doi: 10.1093/hmg/8.7.1321. [DOI] [PubMed] [Google Scholar]
- 7.Hudspeth A. Curr Opin Neurobiol. 1997;7:480–486. doi: 10.1016/s0959-4388(97)80026-8. [DOI] [PubMed] [Google Scholar]
- 8.Matsubara A, Laake J H, Davanger S, Usami S, Ottersen O P. J Neurosci. 1996;16:4457–4467. doi: 10.1523/JNEUROSCI.16-14-04457.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petralia R S, Zhao H M, Wang Y X, Wenthold R J. Neuropharmacology. 1998;37:1321–1334. doi: 10.1016/s0028-3908(98)00118-x. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y X, Wenthold R J, Ottersen O P, Petralia R S. J Neurosci. 1998;18:1148–1160. doi: 10.1523/JNEUROSCI.18-03-01148.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartmann D, Fehr S, Meyerhof W, Richter D. Dev Neurosci (Basel) 1995;17:246–255. doi: 10.1159/000111293. [DOI] [PubMed] [Google Scholar]
- 12.Lim D J, Anniko M. Acta Otolaryngol Suppl. 1985;422:1–69. [PubMed] [Google Scholar]
- 13.Usami S, Hozawa J, Shinkawa H, Saito S, Matsubara A, Fujita S. Acta Otolaryngol Suppl. 1993;506:7–13. doi: 10.3109/00016489309130231. [DOI] [PubMed] [Google Scholar]
- 14.Lysakowski A, Goldberg J M. J Comp Neurol. 1997;389:419–443. doi: 10.1002/(sici)1096-9861(19971222)389:3<419::aid-cne5>3.0.co;2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wersäll R A, Bagger-Sjöbäck D. In: Handbook of Sensory Physiology. Vestibular System. Basic Mechanisms. Kornhuber H H, editor. New York: Springer; 1974. pp. 123–170. [Google Scholar]
- 16.Lingenhohl K, Friauf E. J Neurosci. 1994;14:1176–1194. doi: 10.1523/JNEUROSCI.14-03-01176.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee Y, Lopez D E, Meloni E G, Davis M. J Neurosci. 1996;16:3775–3789. doi: 10.1523/JNEUROSCI.16-11-03775.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakagawa T, Kakehata S, Yamamoto T, Akaike N, Komune S, Uemura T. Brain Res. 1994;661:293–297. doi: 10.1016/0006-8993(94)91207-6. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg J M. J Neurophysiol. 1996;76:1942–1957. doi: 10.1152/jn.1996.76.3.1942. [DOI] [PubMed] [Google Scholar]
- 20.Rüsch A, Eatock R A. J Neurophysiol. 1996;76:995–1004. doi: 10.1152/jn.1996.76.2.995. [DOI] [PubMed] [Google Scholar]
- 21.Rüsch A, Lysakowski A, Eatock R A. J Neurosci. 1998;18:7487–7501. doi: 10.1523/JNEUROSCI.18-18-07487.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mammano F, Kros C J, Ashmore J F. Pflügers Arch. 1995;430:745–750. doi: 10.1007/BF00386170. [DOI] [PubMed] [Google Scholar]
- 23.Mammano F, Ashmore J F. J Physiol (London) 1996;496:639–646. doi: 10.1113/jphysiol.1996.sp021715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Housley G D, Ashmore J F. J Physiol (London) 1992;448:73–98. doi: 10.1113/jphysiol.1992.sp019030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcotti W, Kros C J. J Physiol (London) 1999;520:653–660. doi: 10.1111/j.1469-7793.1999.00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H S, Pan Z, Shi W, Brown B S, Wymore R S, Cohen I S, Dixon J E, McKinnon D. Science. 1998;282:1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong C E, Roberts W M. J Neurosci. 1998;18:2962–2973. doi: 10.1523/JNEUROSCI.18-08-02962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lennan G W, Steinacker A, Lehouelleur J, Sans A. Pflügers Arch. 1999;438:40–46. doi: 10.1007/s004240050877. [DOI] [PubMed] [Google Scholar]
- 29.Abbott G W, Sesti F, Splawski I, Buck M E, Lehmann M H, Timothy K W, Keating M T, Goldstein S A. Cell. 1999;97:175–187. doi: 10.1016/s0092-8674(00)80728-x. [DOI] [PubMed] [Google Scholar]
- 30.Biervert C, Schroeder B C, Kubisch C, Berkovic S F, Propping P, Jentsch T J, Steinlein O K. Science. 1998;279:403–406. doi: 10.1126/science.279.5349.403. [DOI] [PubMed] [Google Scholar]
- 31.Schroeder B C, Kubisch C, Stein V, Jentsch T J. Nature (London) 1998;396:687–690. doi: 10.1038/25367. [DOI] [PubMed] [Google Scholar]
- 32.Li H, Mizuno N. Neurosci Res (NY) 1997;29:135–142. doi: 10.1016/s0168-0102(97)00082-5. [DOI] [PubMed] [Google Scholar]
- 33.Herbert H, Klepper A, Ostwald J. Anat Embryol. 1997;196:235–259. doi: 10.1007/s004290050094. [DOI] [PubMed] [Google Scholar]
- 34.Moriizumi T, Hattori T. Exp Brain Res. 1991;87:223–226. doi: 10.1007/BF00228524. [DOI] [PubMed] [Google Scholar]
- 35.Olazabal U E, Moore J K. J Comp Neurol. 1989;282:98–118. doi: 10.1002/cne.902820108. [DOI] [PubMed] [Google Scholar]
- 36.Schwarz D W, Schwarz I E. J Otolaryngol. 1992;21:339–342. [PubMed] [Google Scholar]
- 37.Klepper A, Herbert H. Brain Res. 1991;557:190–201. doi: 10.1016/0006-8993(91)90134-h. [DOI] [PubMed] [Google Scholar]
- 38.Trussell L O. Curr Opin Neurobiol. 1997;7:487–492. doi: 10.1016/s0959-4388(97)80027-x. [DOI] [PubMed] [Google Scholar]
- 39.Trussell L O. Annu Rev Physiol. 1999;61:477–496. doi: 10.1146/annurev.physiol.61.1.477. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Kunkel D D, Schwartzkroin P A, Tempel B L. J Neurosci. 1994;14:4588–4599. doi: 10.1523/JNEUROSCI.14-08-04588.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L Y, Gan L, Forsythe I D, Kaczmarek L K. J Physiol (London) 1998;509:183–194. doi: 10.1111/j.1469-7793.1998.183bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fekete D M. Trends Neurosci. 1999;22:263–269. doi: 10.1016/s0166-2236(98)01366-6. [DOI] [PubMed] [Google Scholar]
- 43.Kunst H, Marres H, Huygen P, Ensink R, Van Camp G, Van Hauwe P, Coucke P, Willems P, Cremers C. Laryngoscope. 1998;108:74–80. doi: 10.1097/00005537-199801000-00014. [DOI] [PubMed] [Google Scholar]
- 44.Baloh R W. Lancet. 1998;352:1841–1846. doi: 10.1016/S0140-6736(98)05430-0. [DOI] [PubMed] [Google Scholar]
- 45.Ryan A, Dallos P. Nature (London) 1975;253:44–46. doi: 10.1038/253044a0. [DOI] [PubMed] [Google Scholar]