Abstract
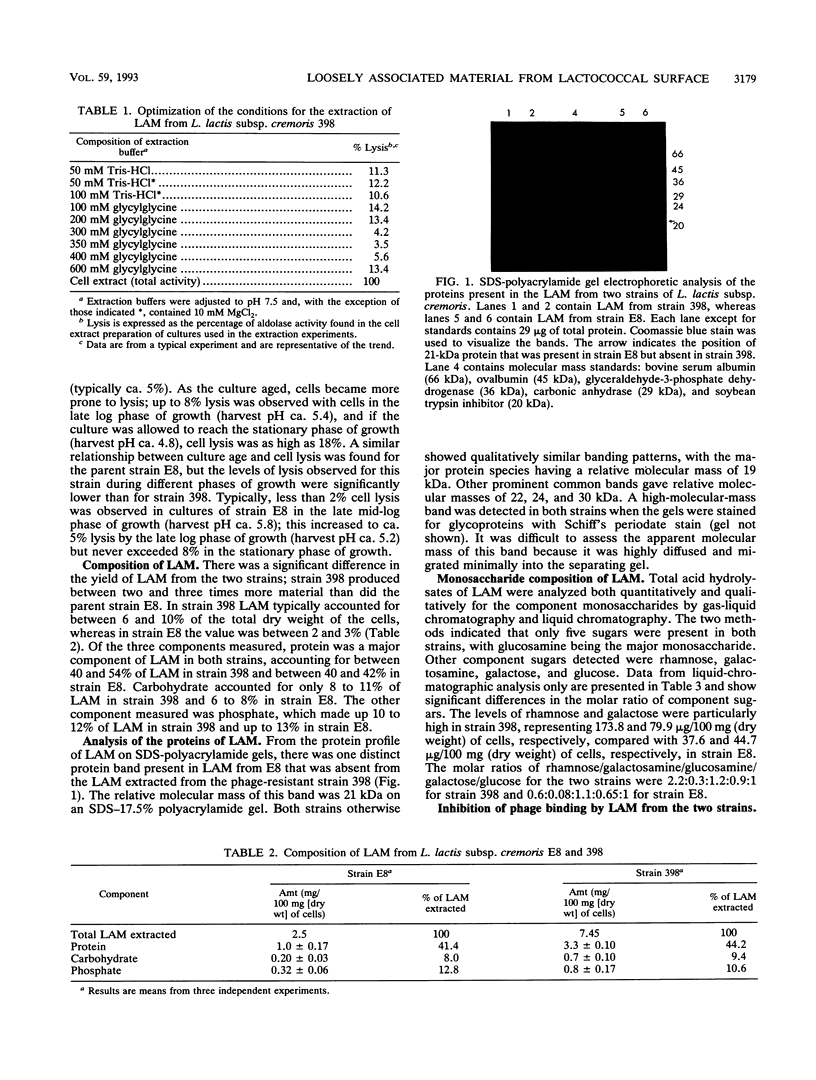
Loosely associated material (LAM) was isolated by gentle extraction procedures from the cell surface of Lactococcus lactis subsp. cremoris E8 and its phage-resistant variant strain 398. LAM from both strains was chemically characterized, and its role in the adsorption of three small isometric bacteriophages, φ 618, φ 833, and φ 852, to the cell surface of the two strains was investigated. The phage-resistant strain (strain 398) produced LAM which differed significantly from the material produced by the parent strain. The total yield of LAM from strain 398 was two- to threefold higher than that from strain E8, and the material contained fivefold more rhamnose and twofold more galactose. Polyacrylamide gel electrophoretic analysis showed that LAM from strain 398 lacked a 21-kDa protein which was present in LAM from the parent strain. Inhibition studies of phage binding by using isolated LAM from two strains showed that although LAM from strain E8 reduced the titer of φ 618 and φ 852 by 53 and 82% respectively, LAM from strain 398 had no effect on the plaque-forming ability of any of the three phages tested. Treatment of LAM from strain E8 with sodium metaperiodate destroyed its ability to bind with φ 618 and φ 852. Phenotypically, strain 398 differed from its parent strain E8 in that it was more prone to cell lysis and required an osmotically adjusted buffer system for the extraction of LAM.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Cleary P. P., Wannamaker L. W., Fisher M., Laible N. Studies of the receptor for phage A25 in group A streptococci: the role of peptidoglycan in reversible adsorption. J Exp Med. 1977 Mar 1;145(3):578–593. doi: 10.1084/jem.145.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow V. L., Thomas T. D. D-tagatose 1,6-diphosphate aldolase from lactic streptococci: purification, properties, and use in measuring intracellular tagatose 1,6-diphosphate. J Bacteriol. 1982 Aug;151(2):600–608. doi: 10.1128/jb.151.2.600-608.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Zabriskie J. B. Studies on streptococcal bacteriophages. II. Adsorption studies on group A and group C streptococcal bacteriophages. J Exp Med. 1968 Mar 1;127(3):489–505. doi: 10.1084/jem.127.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller K. J. Molecular interaction between bacteriophage and the gram-negative cell envelope. Arch Microbiol. 1992;158(4):235–248. doi: 10.1007/BF00245239. [DOI] [PubMed] [Google Scholar]
- Jenkinson H. F., Easingwood R. A. Insertional inactivation of the gene encoding a 76-kilodalton cell surface polypeptide in Streptococcus gordonii Challis has a pleiotropic effect on cell surface composition and properties. Infect Immun. 1990 Nov;58(11):3689–3697. doi: 10.1128/iai.58.11.3689-3697.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McNab R., Jenkinson H. F. Gene disruption identifies a 290 kDa cell-surface polypeptide conferring hydrophobicity and coaggregation properties in Streptococcus gordonii. Mol Microbiol. 1992 Oct;6(20):2939–2949. doi: 10.1111/j.1365-2958.1992.tb01753.x. [DOI] [PubMed] [Google Scholar]
- Oram J. D. Isolation and properties of a phage receptor substance from the plasma membrane of Streptococcus lactis ML 3. J Gen Virol. 1971 Oct;13(1):59–71. doi: 10.1099/0022-1317-13-1-59. [DOI] [PubMed] [Google Scholar]
- Sanders M. E. Phage resistance in lactic acid bacteria. Biochimie. 1988 Mar;70(3):411–422. doi: 10.1016/0300-9084(88)90215-5. [DOI] [PubMed] [Google Scholar]
- Schäfer A., Geis A., Neve H., Teuber M. Bacteriophage receptors of Lactococcus lactis subsp. 'diacetylactis' F7/2 and Lactococcus lactis subsp. cremoris Wg2-1. FEMS Microbiol Lett. 1991 Feb;62(1):69–73. doi: 10.1016/0378-1097(91)90257-b. [DOI] [PubMed] [Google Scholar]
- Sijtsma L., Jansen N., Hazeleger W. C., Wouters J. T., Hellingwerf K. J. Cell Surface Characteristics of Bacteriophage-Resistant Lactococcus lactis subsp. cremoris SK110 and Its Bacteriophage-Sensitive Variant SK112. Appl Environ Microbiol. 1990 Oct;56(10):3230–3233. doi: 10.1128/aem.56.10.3230-3233.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijtsma L., Sterkenburg A., Wouters J. T. Properties of the Cell Walls of Lactococcus lactis subsp. cremoris SK110 and SK112 and Their Relation to Bacteriophage Resistance. Appl Environ Microbiol. 1988 Nov;54(11):2808–2811. doi: 10.1128/aem.54.11.2808-2811.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijtsma L., Wouters J. T., Hellingwerf K. J. Isolation and characterization of lipoteichoic acid, a cell envelope component involved in preventing phage adsorption, from Lactococcus lactis subsp. cremoris SK110. J Bacteriol. 1990 Dec;172(12):7126–7130. doi: 10.1128/jb.172.12.7126-7130.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valyasevi R., Sandine W. E., Geller B. L. A membrane protein is required for bacteriophage c2 infection of Lactococcus lactis subsp. lactis C2. J Bacteriol. 1991 Oct;173(19):6095–6100. doi: 10.1128/jb.173.19.6095-6100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Takesue S. Use of L-rhamnose to study irreversible adsorption of bacteriophage PL-1 to a strain of Lactobacillus casei. J Gen Virol. 1975 Jul;28(1):29–35. doi: 10.1099/0022-1317-28-1-29. [DOI] [PubMed] [Google Scholar]
- Yokokura T. Phage receptor material in Lactobacillus casei. J Gen Microbiol. 1977 May;100(1):139–145. doi: 10.1099/00221287-100-1-139. [DOI] [PubMed] [Google Scholar]
- Young F. E. Requirement of glucosylated teichoic acid for adsorption of phage in Bacillus subtilis 168. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2377–2384. doi: 10.1073/pnas.58.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]