Abstract
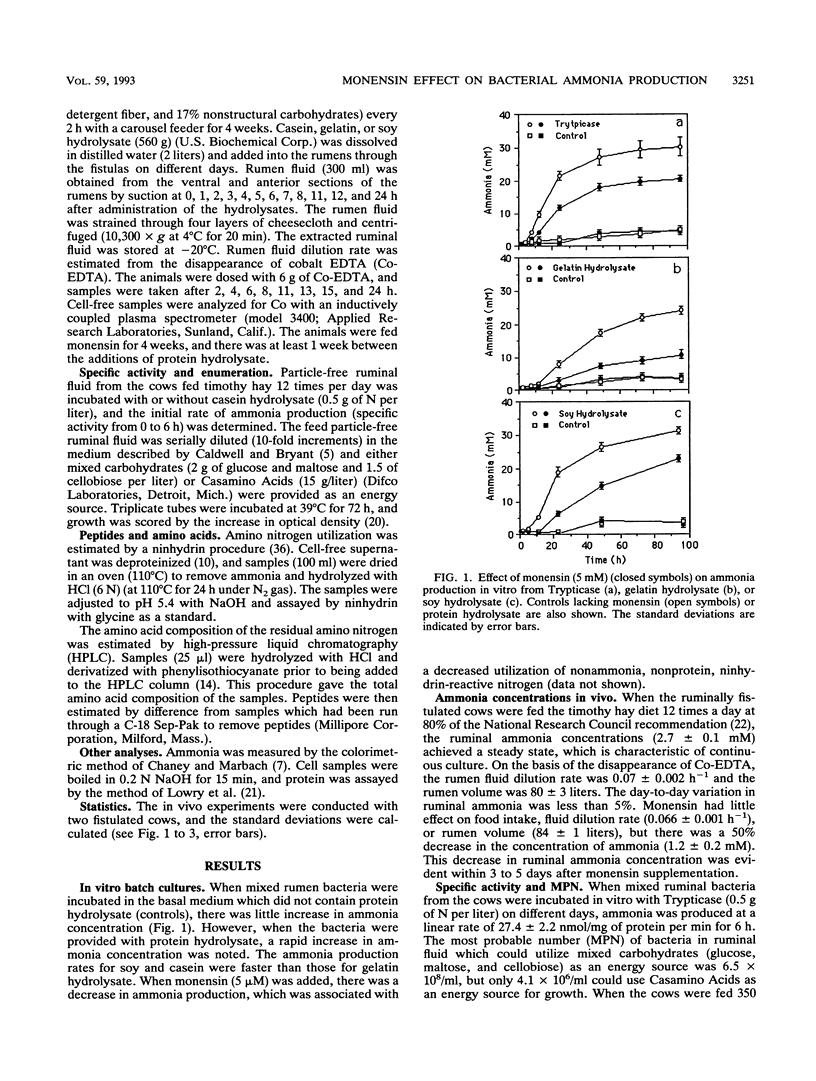
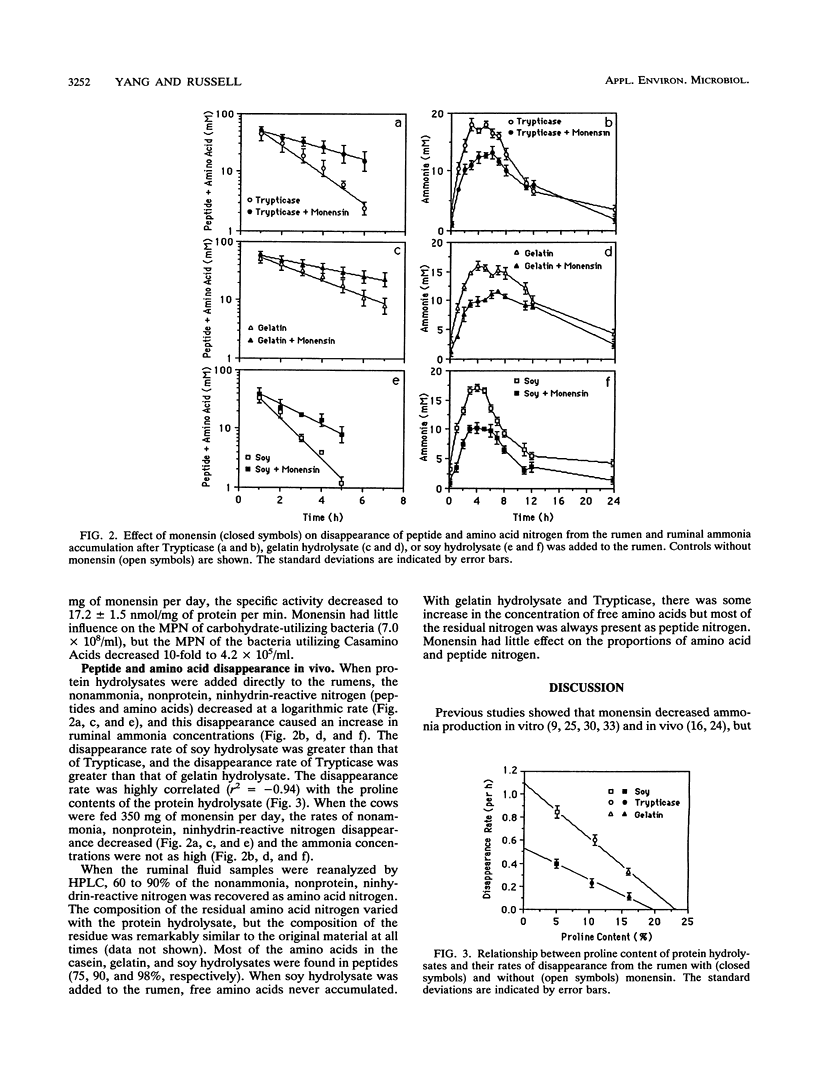
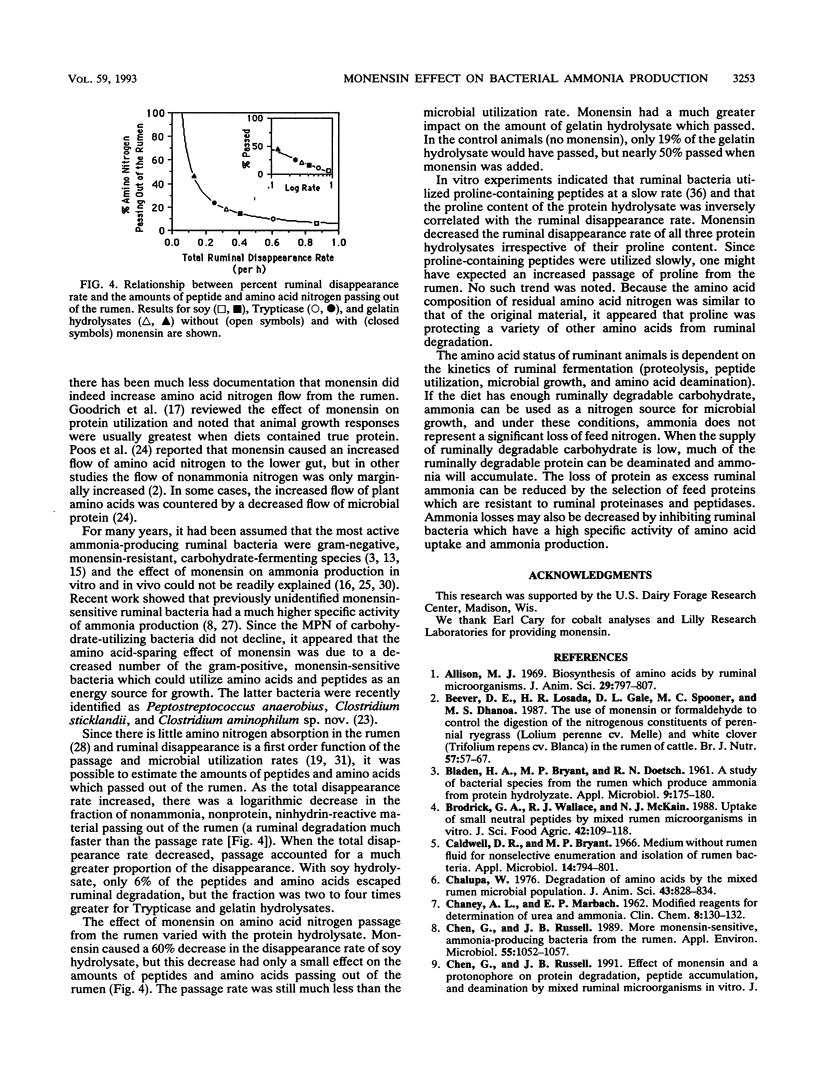
When unadapted mixed ruminal bacteria (312 mg of protein per liter) were treated with monensin (5 mM) in vitro, the rates of ammonia production from enzymatic digests of casein, gelatin, and soy protein (0.5 g of N per liter) were decreased from 46 +/- 2 to 24 +/- 1, 20 +/- 1 to 7 +/- 1, and 40 +/- 2 to 18 +/- 2 nmol/mg of protein per min, respectively. Monensin also caused a decrease in ammonia production in vivo. Nonlactating dairy cows which were fed 0.56 kg of timothy hay 12 times per day had a steady-state ruminal ammonia concentration of 2.7 +/- 0.1 mM, and the ammonia concentration decreased to 1.2 +/- 0.2 mM when monensin (350 mg/day) was added to the diet. The decrease in ammonia production was associated with a 10-fold reduction (4.1 x 10(6) versus 4.2 x 10(5)/ml) in the most probable number of ammonia-producing ruminal bacteria that could use protein hydrolysate as an energy source. Monensin had little effect on the most probable number of carbohydrate-utilizing ruminal bacteria (6.5 versus 7.0 x 10(8)/ml). The addition of protein hydrolysates (560 g) to the rumen caused a rapid increase in the ammonia concentration, but this increase was at least 30% lower when the animals were fed monensin.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison M. J. Biosynthesis of amono acids by ruminal microorganisms. J Anim Sci. 1969 Nov;29(5):797–807. doi: 10.2527/jas1969.295797x. [DOI] [PubMed] [Google Scholar]
- Beever D. E., Losada H. R., Gale D. L., Spooner M. C., Dhanoa M. S. The use of monensin or formaldehyde to control the digestion of the nitrogenous constituents of perennial ryegrass (Lolium perenne cv. Melle) and white clover (Trifolium repens cv. Blanca) in the rumen of cattle. Br J Nutr. 1987 Jan;57(1):57–67. doi: 10.1079/bjn19870009. [DOI] [PubMed] [Google Scholar]
- Bladen H. A., Bryant M. P., Doetsch R. N. A Study of Bacterial Species from the Rumen Which Produce Ammonia from Protein Hydrolyzate. Appl Microbiol. 1961 Mar;9(2):175–180. doi: 10.1128/am.9.2.175-180.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANEY A. L., MARBACH E. P. Modified reagents for determination of urea and ammonia. Clin Chem. 1962 Apr;8:130–132. [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalupa W. Degradation of amino acids by the mixed rumen microbial population. J Anim Sci. 1976 Oct;43(4):828–834. doi: 10.2527/jas1976.434828x. [DOI] [PubMed] [Google Scholar]
- Chen G., Russell J. B. More monensin-sensitive, ammonia-producing bacteria from the rumen. Appl Environ Microbiol. 1989 May;55(5):1052–1057. doi: 10.1128/aem.55.5.1052-1057.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Russell J. B., Sniffen C. J. A procedure for measuring peptides in rumen fluid and evidence that peptide uptake can be a rate-limiting step in ruminal protein degradation. J Dairy Sci. 1987 Jun;70(6):1211–1219. doi: 10.3168/jds.S0022-0302(87)80133-9. [DOI] [PubMed] [Google Scholar]
- Chen G., Sniffen C. J., Russell J. B. Concentration and estimated flow of peptides from the rumen of dairy cattle: effects of protein quantity, protein solubility, and feeding frequency. J Dairy Sci. 1987 May;70(5):983–992. doi: 10.3168/jds.S0022-0302(87)80103-0. [DOI] [PubMed] [Google Scholar]
- Chen G., Strobel H. J., Russell J. B., Sniffen C. J. Effect of hydrophobicity of utilization of peptides by ruminal bacteria in vitro. Appl Environ Microbiol. 1987 Sep;53(9):2021–2025. doi: 10.1128/aem.53.9.2021-2025.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Wolin M. J. Effect of monensin and lasalocid-sodium on the growth of methanogenic and rumen saccharolytic bacteria. Appl Environ Microbiol. 1979 Jul;38(1):72–77. doi: 10.1128/aem.38.1.72-77.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis S. M., Nagaraja T. G., Bartley E. E. Effects of lasalocid or monensin on lactate-producing or -using rumen bacteria. J Anim Sci. 1981 Feb;52(2):418–426. doi: 10.2527/jas1981.522418x. [DOI] [PubMed] [Google Scholar]
- Donius D. A., Simpson M. E., Marsh P. B. Effect of monensin fed with forage on digestion and the ruminal ecosystem of steers. J Anim Sci. 1976 Jan;42(1):229–234. doi: 10.2527/jas1976.421229x. [DOI] [PubMed] [Google Scholar]
- Goodrich R. D., Garrett J. E., Gast D. R., Kirick M. A., Larson D. A., Meiske J. C. Influence of monensin on the performance of cattle. J Anim Sci. 1984 Jun;58(6):1484–1498. doi: 10.2527/jas1984.5861484x. [DOI] [PubMed] [Google Scholar]
- Hino T., Russell J. B. Relative contributions of ruminal bacteria and protozoa to the degradation of protein in vitro. J Anim Sci. 1987 Jan;64(1):261–270. doi: 10.2527/jas1987.641261x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Paster B. J., Russell J. B., Yang C. M., Chow J. M., Woese C. R., Tanner R. Phylogeny of the ammonia-producing ruminal bacteria Peptostreptococcus anaerobius, Clostridium sticklandii, and Clostridium aminophilum sp. nov. Int J Syst Bacteriol. 1993 Jan;43(1):107–110. doi: 10.1099/00207713-43-1-107. [DOI] [PubMed] [Google Scholar]
- Poos M. I., Hanson T. L., Klopfenstein T. J. Monensin effects on diet digestibility, ruminal protein bypass and microbial protein synthesis. J Anim Sci. 1979 Jun;48(6):1516–1524. doi: 10.2527/jas1979.4861516x. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Sniffen C. J., Van Soest P. J. Effect of carbohydrate limitation on degradation and utilization of casein by mixed rumen bacteria. J Dairy Sci. 1983 Apr;66(4):763–775. doi: 10.3168/jds.S0022-0302(83)81856-6. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Strobel H. J., Chen G. J. Enrichment and isolation of a ruminal bacterium with a very high specific activity of ammonia production. Appl Environ Microbiol. 1988 Apr;54(4):872–877. doi: 10.1128/aem.54.4.872-877.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nevel C. J., Demeyer D. I. Effect of monensin on rumen metabolism in vitro. Appl Environ Microbiol. 1977 Sep;34(3):251–257. doi: 10.1128/aem.34.3.251-257.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo D. R., Smith L. W., Cox E. L. Model of cellulose disappearance from the rumen. J Dairy Sci. 1972 Jan;55(1):125–129. doi: 10.3168/jds.S0022-0302(72)85442-0. [DOI] [PubMed] [Google Scholar]
- Whetstone H. D., Davis C. L., Bryant M. P. Effect of monensin on breakdown of protein by ruminal microorganisms in vitro. J Anim Sci. 1981 Sep;53(3):803–809. doi: 10.2527/jas1981.533803x. [DOI] [PubMed] [Google Scholar]
- Wright D. E., Hungate R. E. Amino acid concentrations in rumen fluid. Appl Microbiol. 1967 Jan;15(1):148–151. doi: 10.1128/am.15.1.148-151.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. M., Russell J. B. Resistance of proline-containing peptides to ruminal degradation in vitro. Appl Environ Microbiol. 1992 Dec;58(12):3954–3958. doi: 10.1128/aem.58.12.3954-3958.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


