Abstract
There is increasing evidence that oxygen free radicals contribute to ischemic brain injury. It is unclear, however, to what extent specific antioxidant enzymes can prevent or reverse the impairment of synaptic function caused by transient hypoxia. In this study, we investigated in transgenic (Tg) mice whether a moderate increase in glutathione peroxidase-1 (GPx1) may improve the capacity of CA1 pyramidal cells to recover synaptic transmission after a short period of hypoxia in vitro. In control hippocampal slices, transient hypoxia (7–9 min) produced irreversible loss of excitatory postsynaptic potentials. Complete recovery of synaptic transmission was observed with homozygous Tg-MT-GPx-6 mice after reoxygenation, and, after repeated episodes of hypoxia, synaptic transmission was still viable in most Tg slices, in contrast to non-Tg slices. Moreover, hypoxic episodes abolished the capacity of hippocampal slices to generate long-term potentiation in area CA1 of control mice, whereas a significant extent of long-term potentiation expression was still preserved in Tg tissues. We also demonstrated that susceptibility to N-methyl-d-aspartate-mediated oxidative injury was reduced in Tg hippocampal slices. In conclusion, our results suggest that a moderate GPx increase can be sufficient to prevent irreversible functional damage produced by transient hypoxia in the hippocampus and to help maintain basic electrophysiological mechanisms involved in memory formation.
There is strong evidence that generation of oxygen free radicals plays a central role in neuronal injury and neurodegeneration induced by transient focal ischemia (see refs. 1 and 2 for reviews). The most noxious radical species implicated is thought to be the hydroxyl radical (⋅OH), which is known to react extremely rapidly with most biological molecules, including DNA, proteins, and, in particular, polyunsaturated fatty acids of membrane lipids. The latter reaction can initiate lipid peroxidation and cascades of free radical-generating reactions that disrupt membrane integrity and trigger calcium-ion overflow into the cell. The mechanisms by which hydroxyl radicals are generated in response to an ischemic insult are not well understood, but it has been suggested that cell injury may be induced by at least two sources of hydroxyl radicals: the decomposition of peroxynitric acid, formed by reaction of nitric oxide (NO) with superoxide (O2−), and metal-dependent Fenton-type reactions involving hydrogen peroxide (H2O2).
By concerted detoxification of superoxide radicals, H2O2, and organic hydroperoxides, cellular antioxidant enzymes can help prevent the formation of very toxic hydroxyl and alkoxyl radicals. In the brain, the most abundant antioxidant enzymes are Cu,Zn- and Mn-superoxide dismutases (SODs), which catalyze the dismutation of O2⨪ to H2O2, and seleno glutathione peroxidase-1 (GPx1), which further converts H2O2 into water and reduces fatty acid hydroperoxides (see review in ref. 3). Transgenic (Tg) mice, which overexpress human SOD-I, were shown to be protected partially against transient (4, 5) but not permanent focal cerebral ischemia (6). Moreover, treatment of hippocampal slices with the SOD-mimic molecule Eukarion-8 conferred significant protection of synaptic function in area CA1 of the hippocampus after a transient hypoxic episode (7). In addition, the reports that both neuronal nitric oxide synthase-knockout mice and Tg-CuZn-SOD mice are partially resistant to cerebral ischemia and that double-Tg animals may even be more protected strongly suggest a contribution of hydroxyl radicals resulting from concomitant induction of NO and superoxide in the infarct area (2).
The contribution of hydroxyl radicals eventually formed by Fenton-type reactions after transient cerebral ischemia is not known, but efficient removal of excess H2O2 by GPx could be another important mechanism that helps to minimize the formation of hydroxyl radicals and oxidative injury. We have shown that GPx1 overexpression can confer protection against iron-dependent O2−/H2O2-mediated DNA damage and cytotoxicity to human cells in vitro (refs. 8 and 9; D.F., P. Petrov, G. Poirier, and M.-E.M., unpublished work). Moreover, studies with the GPx mimic Ebselen have demonstrated that morphological damage elicited by brain ischemia is markedly reduced in the rat brain (10). Recently, Tg mice overexpressing GPx1 were found to be partially resistant to myocardial ischemia (11) and, more recently, to brain ischemia (12). However, these Tg mice, with high levels of GPx in the brain, may show abnormal physiological responses, such as a thermosensitive phenotype (13). On the other hand, it is not known whether the protective effects observed at the morphological level in response to focal cerebral ischemia (12) are also exerted at the functional level.
The deleterious effects of transient hypoxia on synaptic function are well documented, and electrophysiological investigations in vitro that use hippocampal slices as an experimental model have revealed that an irreversible reduction of synaptic transmission in area CA1 can occur rapidly after a short period of hypoxia (7, 14). It also was shown that hypoxic episodes severely disrupt the mechanisms that produce long-term potentiation (LTP) in area CA1 of hippocampal slices (15). This observation is of particular interest because LTP seems to represent a basic mechanism used in many neural networks to store various types of information (16). The aim of the present study was to evaluate whether a moderate increase of GPx expression in area CA1 of the mouse hippocampus may provide substantial protection to basal synaptic transmission as well as LTP maintenance after a transient hypoxic insult in vitro.
Materials and Methods
Tg Mice.
Tg mice (B6C3F1), hemizygous or homozygous for the integration of 1–3 copies of human GPx1 cDNA transgenes under the control of a metallothionein (MT) promoter, were produced and bred as described previously (9). Unless specified, the data reported here were obtained with homozygous Tg-MT-GPx-6 mice and compared with non-Tg animals of the same strain. Some experiments were also carried out with a second line of Tg mice, MT-GPx-13. Tg mice were identified by Southern analysis of tail DNA (9).
GPx Expression and Activity.
Immunocytochemical analysis of GPx expression in the mouse hippocampal region was performed as described elsewhere (17) by using an immunoaffinity-purified antibody derived from polyclonal antiserum raised against recombinant human GPx1. GPx activity was measured by coupled assay with t-butyl hydroperoxide as substrate, as previously described (18). In brief, hippocampi were homogenized in ice-cold 0.1 M potassium phosphate buffer, pH 7.8, containing 1 mM EDTA, 0.5 μg/ml leupeptin, 0.5 μg/ml pepstatin A, 17 μg/ml phenylmethylsulfonyl fluoride using a Kontes homogenizer (pestle SZ 20). Triton X-100 (2% final concentration) was added to the homogenates for 1 h at 4°C, and the samples were centrifuged to remove insoluble materials. GPx activity was assayed in the supernatants, and specific activity is expressed as nmol of NADPH oxidized per min at 37°C (milliunit) per mg of protein.
Electrophysiology.
Experiments were performed on hippocampal slices (450 μm thick) prepared from control and Tg mice as described previously (19). The slices were exposed to a humidified atmosphere of 95% O2/5% CO2 and perfused continuously at a flow rate of 1 ml/min, unless otherwise stated. After a 1-h equilibration period, a glass recording electrode (1–5 MΩ; filled with 2 M NaCl) was positioned in the stratum radiatum of area CA1 to record population excitatory postsynaptic potentials (EPSPs) evoked by a bipolar electrode activating fibers of the Schaffer commissural system. All field EPSPs were allowed to stabilize in oxygenated artificial cerebrospinal fluid (ACSF) for the initial 15–20 min of recording, a period during which responses were registered every 30 sec. Episodes of hypoxia were induced by replacing the oxygen supply with 100% N2 gas delivered at the same pressure. N2 was administered for a duration that outlasted the loss of fiber volley for 90 sec, after which oxygenation was resumed. In some experiments, high-frequency stimulation [10 bursts of four stimulations at 100 Hz given at 200-msec intervals, theta burst stimulation (TBS)] was applied to generate synaptic potentiation in area CA1 of the hippocampus. The response to stimulation was quantified by calculating the initial slope of the resulting EPSP. Most comparisons between groups were made by using a one-way analysis of variance (ANOVA) followed by the Bonferroni t test.
Oxidative Stress Indices.
Formation of thiobarbituric acid reactive substances (TBARS) in hippocampus was assessed in tissue extracts incubated for 15 min in the absence or in the presence of Fe2+ and ascorbic acid to stimulate H2O2-dependent hydroxyl radical formation and lipid peroxidation, as described by Barrier et al. (20). Protein oxidation in these extracts was assessed by a Western blotting carbonyl assay using a monoclonal antibody to 2,4-dinitrophenyl moiety (Sigma) (21). Integrated optical densities of the major protein band signals were normalized to actin content assessed on the same blot with a monoclonal antibody to β-actin (Sigma).
Results
Hippocampal Expression of GPx in Tg-GPx1 Mice.
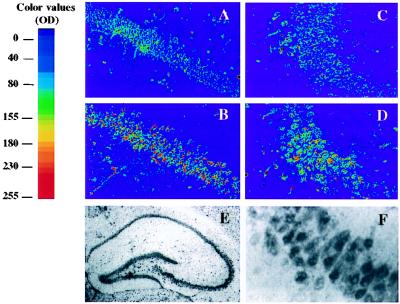
Both Northern and Western blot analyses have shown previously that GPx expression in Tg-MT-GPx-6 mice occurs in most brain regions, including the substantia nigra, the striatum, the cortex, and the cerebellum; the corresponding increase of GPx activity measured in the mesencephalon, for example, was ≈50% (9). When GPx activity was assayed in single hippocampus extracts (in this study), homozygous Tg-MT-GPx (+/+) mice presented only a minor increase in global GPx activity as compared with control non-Tg mice (line Tg-MT-GPx-6, 39 ± 2.4 milliunits/mg versus 34.2 ± 1.2 milliunits/mg in controls; n = 6; P < 0.001; line Tg-MT-GPx-13, 42.4 ± 3.2 milliunits/mg; n = 7; P < 0.0001). The possibility that the activity increase was unevenly distributed in the hippocampal region, i.e., that GPx expression was augmented selectively in specific areas and cell types, was investigated by immunocytochemistry. Fig. 1 shows the immunostaining obtained with immunoaffinity-purified anti-human GPx1 IgG in areas CA1 (A and B) and CA3 (C and D) of the hippocampus in non-Tg (A and C) and homozygous Tg-MT-GPx-6 (+/+) mice (B and D); this antibody reacts with both human and mouse GPx (9). As shown in Fig. 1 E and F, GPx-like immunoreactivity in CA1 was strongly enriched in the pyramidal cell layer of the hippocampus. High magnification of the CA1 segment indeed revealed intense GPx-like staining in pyramidal cells (Fig. 1F). As can be seen in Fig. 1, there is increased staining in the pyramidal cell layer of area CA1 of the Tg hippocampus (Fig. 1B) as compared with the corresponding region in non-Tg mice (Fig. 1A). Staining was also more intense in area CA3 of the hippocampus in the Tg brain (Fig. 1 C and D) as well as in the dentate gyrus (data not shown). A very similar pattern of enhanced GPx enrichment in the hippocampal pyramidal cell layer was found in a second Tg line, Tg-MT-GPx-13 (data not shown). As demonstrated previously (17), appropriate controls showed no detectable immunostaining with preimmune antibody or when the primary antibody was omitted, and staining was largely suppressed when the antibody was preadsorbed with commercial GPx. Therefore, the localized increase of immunoreactivity observed in the pyramidal cell layer of the Tg hippocampus was specific to the GPx antigen. On the other hand, we found no change in the levels of the other antioxidant enzymes Cu,Zn-SOD and catalase in both Tg lines (data not shown).
Figure 1.
GPx1-like immunoreactivity in areas CA1 and CA3 of hippocampal sections prepared from Tg-GPx mice. Pseudocolor representations of immunoreactivity in areas CA1 (A and B) and CA3 (C and D) of the hippocampus of non-Tg (A and C) and Tg-MT-GPx-6 (+/+) (B and D) mice. Photomicrographs A and C show GPx-like immunostaining in areas CA1 and CA3 of non-Tg mice, respectively. Note the more intense staining (red shift) in the cell body layer in both the CA1 and CA3 regions in Tg mouse as compared with non-Tg mouse. (E) GPx-like staining in the whole hippocampal region of the Tg mouse (low magnification; ×20). (F) High-magnification (×400) picture of a CA1 segment of hippocampal section shown in E, showing intense staining in the pyramidal cells.
Recovery of Synaptic Transmission After Transient Hypoxia Is Increased in Tg-GPx1 Mouse Hippocampus.
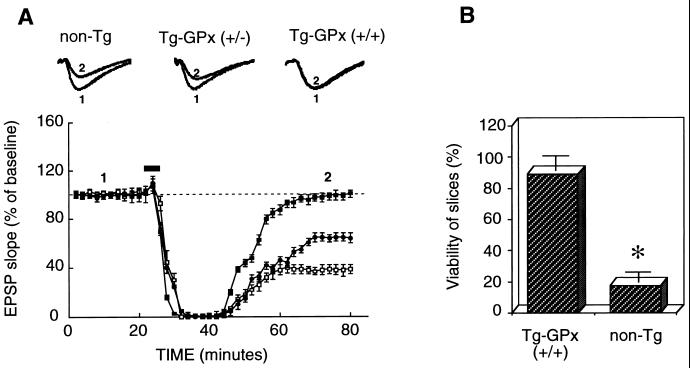
Electrophysiological experiments were performed to determine whether the increased GPx expression detected in the Tg hippocampus was sufficient to exert protective effects on neuronal function after transient hypoxic insults. To this end, hippocampal slices were prepared and perfused for at least 60 min before delivering electrical stimulation to the Schaffer collateral inputs to area CA1 of the hippocampus. After 20 min of baseline recording, the oxygen supply was replaced by nitrogen for a duration that outlasted the complete elimination of EPSPs by 90 sec. As shown in Figure 2A, hypoxia initially caused complete depression of synaptic responses in hippocampal slices, regardless of GPx Tg status. In contrast, however, recovery of synaptic responses after reoxygenation was remarkably more efficient in Tg slices than in non-Tg controls (Fig. 2A, open squares). This result was clearly visible at the levels of both initial rate and extent of EPSP slope recovery in slices prepared from Tg-MT-GPx-6 mice that were homozygotes for human GPx1 (+/+) (Fig. 2A, filled squares). EPSP slope recovery essentially was complete 40 min after reoxygenation (slope average, 98 ± 5% of baseline value in Tg mice vs. 38 ± 8% of baseline value for non-Tg controls; n = 8; P < 0.01). Similar analysis was performed in Tg-MT-GPx-6 mice hemizygotes (+/−) for human GPx1. Again, these mice showed a significantly better, although partial, degree of recovery from hypoxia-induced synaptic impairment (Fig. 2A, filled circles). Recovery of EPSPs in these mice (slope average, 65 ± 5% of baseline value; n = 6; P < 0.01) was about intermediate between that of homozygous Tg mice and of non-Tg controls. Moreover, extensive, although partial, recovery of EPSPs was also observed in hippocampal slices of a second Tg line, MT-GPx-13, homozygous for the presence of GPx1 transgenes (slope average, 76 ± 5% of baseline value; n = 5; P < 0.05; data not shown). It should be noted that there were no significant differences in magnitude and shape of EPSPs during the baseline period between control and Tg mice (see EPSP traces in Fig. 2A).
Figure 2.
Recovery of synaptic transmission in Tg hippocampal slices after transient exposure to hypoxia. (A) Hippocampal slices were incubated in ACSF, and EPSPs were elicited by stimulation of the Schaffer commissural pathway in area CA1 of the hippocampus. The duration of hypoxia outlasted the loss of fiber volley for 90 sec before oxygenation was resumed. The data represent the slope of the EPSP and are expressed as percentages of the average of the EPSP slope during the 20-min baseline period (mean ± SEM of 13 experiments). Open squares, slices from non-Tg mice; filled squares, slices from Tg-GPx mice (+/+); filled circles, slices from hemizygous Tg-GPx mice (+/−). The horizontal bars indicate the time during which hypoxia was applied. EPSP traces recorded before (1) and 40 min after (2) the hypoxic episode in both Tg and control slices are shown above the graph. Each sample trace is an average of 10 individual responses. (B) Fifty minutes after two episodes of hypoxia, EPSPs were elicited in hippocampal slices from non-Tg mice and Tg-GPx mice (+/+) by increasing stimulation intensity. A slice was defined as viable if an EPSP with an amplitude of at least 3.0 mV could be elicited. Data represent the percentage of slices viable in each group after the second hypoxic episode (mean ± SEM of six experiments; n = 18 in each group). The difference in viability was statistically significant (P < 0.01).
In addition, the “functional viability” of hippocampal slices after several hypoxic episodes was evaluated according to the criteria used by Arai et al. (22). In brief, slices in which an EPSP with an amplitude of at least 3.0 mV could be evoked by stimulation of the Schaffer collateral pathway were defined as viable. With this definition, most hippocampal slices prepared from homozygous Tg-MT-GPx-6 mice (+/+) were found to be resistant to a second hypoxic episode (89 ± 7%), in contrast to slices from non-Tg mice (17 ± 7%; P < 0.01; Fig. 2B).
Protection of LTP in Tg-GPx1 Mouse Hippocampus.
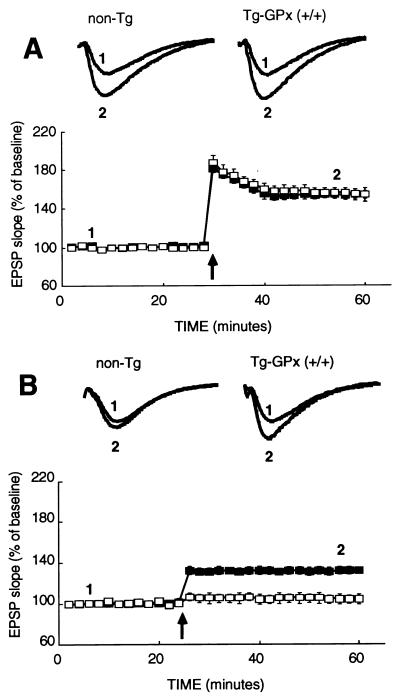
Previous electrophysiological investigations have shown that hypoxic episodes severely disrupt the mechanisms that produce LTP in area CA1 of the hippocampus (15). Thus, experiments were performed to determine whether increased GPx activity might prevent loss of a LTP response in the CA1 region. The capacity of hippocampal slices to generate synaptic potentiation was compared in homozygous Tg-MT-GPx-6 (+/+) and non-Tg mice, both before and after a transient hypoxic episode. Hippocampal LTP was elicited by applying high-frequency stimulation to the Schaffer commissural pathway of area CA1. Four evoked responses were averaged 2 and 30 min after TBS to evaluate the effect of GPx overexpression on short-term potentiation and LTP, respectively. As shown in Fig. 3A, the magnitude of both short-term potentiation (75 ± 5% in control vs. 75 ± 6% in Tg mice; n = 13) and LTP (55 ± 5% in controls vs. 55 ± 6% in Tg mice; n = 14) was similar in Tg and non-Tg animals before any hypoxic episode. As can be seen in Fig. 3B, hypoxia abolished the capacity of area CA1 to generate LTP in control non-Tg slices (7 ± 5%; n = 12). In contrast, hippocampal slices from Tg mice still produced a significant degree of LTP (37 ± 3%; n = 12) after hypoxic exposure. This result suggests that the increase of GPx activity in the Tg hippocampus was also associated with partial protection of synaptic potentiation capacity against hypoxic injury.
Figure 3.
LTP responses in Tg-GPx mouse hippocampal slices after transient hypoxia. Hippocampal slices were incubated in ACSF, and EPSPs were elicited by stimulation of the Schaffer commissural pathway in area CA1 of the hippocampus. (A) Measurements before hypoxic episodes. In control slices (open squares), the slope of the response exhibited ≈80% potentiation after TBS, which slowly decayed during the first 10 min; the potentiation remained stable at 55% afterward. Tg mice (filled squares) show LTP with a similar time course and magnitude to those of control mice. (B) Slices were allowed to recover for 1 h after a hypoxic episode as in Fig. 2. In control slices (open squares), the slope of the response exhibited no LTP (7 ± 4%) after TBS. A substantial degree of LTP (37 ± 3%) was observed in Tg slices homozygous for GPx (+/+) (filled squares) after one hypoxic episode. EPSP traces recorded before (1) and 40 min after (2) TBS in both Tg and control slices are presented above the graphs. Each sample trace is an average of 10 individual responses.
Effects of GPx Inhibition by Mercaptosuccinate (MS) on Hippocampal Synaptic Transmission Recovery in Tg-GPx1 Mouse.
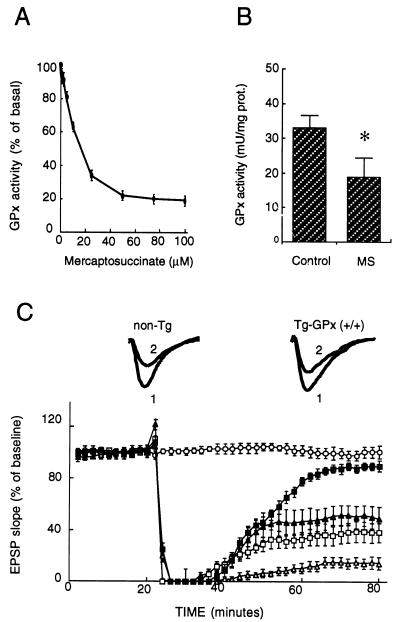
The data presented so far suggest that the Tg phenotypes described above are linked to GPx1 overexpression. To further test this apparent link, we examined the effects of a specific GPx inhibitor, MS, on recovery of synaptic transmission after a transient hypoxia, using the same protocol as described in Fig. 2. MS is a potent reversible inhibitor that binds to selenium in the active site of GPx1 in competition with glutathione (23). If our hypothesis is correct, this inhibitor would be expected to abolish the improved recovery of synaptic transmission in the Tg mice and to decrease the normal extent of recovery observed in control non-Tg mice. To test this hypothesis, we first had to determine the concentration of MS to be used to achieve efficient GPx inhibition. Fig. 4A shows the MS concentration-dependent inhibition of GPx1 measured in mouse hippocampal homogenates, indicating a maximal inhibition of 80% at MS concentration >50 μM. Fig. 4B shows that perfusion of hippocampal slices with 75 μM MS in ACSF medium resulted in an apparent 45 ± 5% (n = 7; P < 0.05) inhibition of GPx. However, the real extent of inhibition in the tissue was likely to be more important because the competitive inhibitor was diluted 10-fold in the extracts prepared for GPx assay. Fig. 4C shows the effects of perfusing hippocampal slices in the presence of 75 μM MS on the EPSP response that were obtained in an experiment similar to that described in Fig. 2 (with no inhibitor). Note that perfusion of hippocampal slices with an ACSF medium containing 75 μM MS did not significantly alter basal synaptic transmission during the course of experiments (Fig. 4C, open circles) in contrast to excessive concentrations above 100 μM (data not shown).
Figure 4.
Effects of GPx inhibition on synaptic transmission recovery after hypoxic exposure. (A) Dose-dependent inhibition of GPx activity by MS added to mouse hippocampal homogenates (non-Tg). Each data point is the mean of two independent measurements. (B) GPx activity determined in Tg-GPx (+/+) hippocampal homogenates from slices treated or not (control) with 75 μM MS (n = 7; P < 0.05). (C) Synaptic responses measured as EPSP slope in area CA1 of hippocampal slices after a hypoxic exposure as described in Fig. 2, but 1 min shorter, in the presence of 75 μM MS. Non-Tg slices (open squares) and Tg slices (MT-GPx-6; filled triangles) are compared with Tg slices without MS (filled squares). MS-treated non-Tg slices after a hypoxic episode that outlasted by 90 sec the loss of fiber volley as in Fig. 2 (open triangles). The data are represented as mean ± SEM of 11 experiments. EPSP traces recorded in MS-treated non-Tg and Tg slices before (1) and 60 min after (2) a 1-min shorter hypoxic episode are shown above the graph. Each sample trace is an average of 10 individual responses.
After hypoxic exposure, two major observations confirmed our expectations. First of all, the GPx inhibitor decreased the recovery of EPSP slope from ≈40% in control non-Tg slices (Fig. 2A, open squares) to ≈10% in corresponding MS-treated slices 40 min after reoxygenation (Fig. 4C, open triangles). Shortening the hypoxia episode by 1 min restored the EPSP slope recovery to ≈40 ± 5% in slices perfused with 75 μM MS (Fig. 4C, open squares). Second, and remarkably, a similar extent of recovery (52 ± 6%; nonsignificant difference) was observed in MS-treated Tg-MT-GPx-6 slices exposed to hypoxia under the same conditions (Fig. 4C, filled triangles), in contrast to the nearly full recovery obtained in Tg-slices in the absence of inhibitor (Fig. 4C, filled squares). This latter data demonstrates that the GPx inhibitor almost abolished the differential recovery of synaptic transmission that was found in Tg-GPx slices. Thus, the MS results strongly support our interpretation that the improved recovery of synaptic transmission in the Tg-MT-GPx mouse hippocampus model after hypoxic insult (Fig. 2) was mainly caused by increased GPx activity.
N-methyl-d-aspartate (NMDA)-Mediated Oxidative Stress Is Attenuated in Tg-GPx1 Mouse Hippocampus.
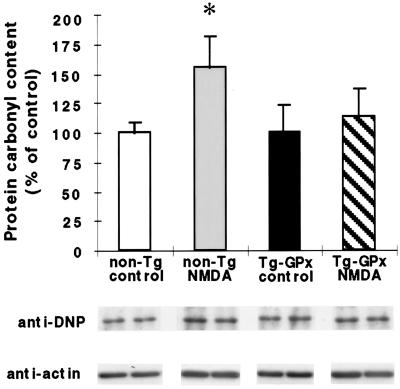
Oxidative stress induced by glutamate-mediated activation of NMDA receptors has been implicated in excitotoxicity (24) and ischemic/hypoxic injury (25, 26). Therefore, experiments were performed to determine whether oxidative stress could be detected in hippocampal slices in response to transient hypoxic exposure or sustained exposure to NMDA. Oxidative stress was assessed in two ways: by protein carbonyl and TBARS formation in hippocampal homogenates (see Materials and Methods). No significant difference in protein carbonyl or rate of TBARS formation could be detected between hippocampal slices exposed or not to hypoxia (10 min or 30 min after a 5-min hypoxic episode; data not shown). The differential reactive oxygen species formation/oxidation induced by transient hypoxia was apparently too low to be detected by either method in this model. In contrast, after a 1-h exposure of hippocampal slices to NMDA, susceptibility to protein oxidation assessed by carbonyl formation was significantly increased in non-Tg slices (51 ± 15%; P < 0.05) as compared with unexposed controls (Fig. 5). However, no significant difference in protein carbonyl formation was detected in Tg-GPx hippocampal homogenates after 1-h NMDA exposure. TBARS formation in the NMDA extracts was also lower in the Tg mice than in non-Tg animals (data not shown).
Figure 5.
Susceptibility to NMDA-mediated protein oxidation is reduced in Tg-GPx mouse hippocampal slices. Hippocampal slices of non-Tg and Tg-GPx mice were incubated for 1 h in the presence or not (controls) of 200 μM NMDA. Protein carbonyl contents were measured by Western blotting of hippocampal extracts incubated as described in Materials and Methods. Carbonyl contents were normalized to actin contents and expressed as percentage of unexposed controls. Mean ± SEM; n = 4 different mice for each group. ∗, Statistically significant difference from control (P < 0.05). The Western blots below the histogramshow corresponding signals from two mice per group.
Discussion
Hippocampal damage has long been recognized as a complicating factor of stroke, with several hypotheses explaining the vulnerability of the hippocampus to ischemic episodes (27–29). A considerable body of information supports the occurrence and pathophysiological importance of free radicals in acute cerebral damage secondary to traumatic and ischemic injury (i.e., stroke) (7, 28, 30, 31). In recent years, several Tg mouse models were used to decipher the roles of individual antioxidant enzymes in this and other pathological conditions thought to involve oxidative stress. In the present investigation, we found that recovery from loss of hippocampal synaptic transmission after transient hypoxic insults in vitro was remarkably improved in Tg mice that overexpress human GPx1 to modest extents in the hippocampus. The effect seems to depend on transgene expression, because the magnitude of synaptic transmission recovery in hemizygous Tg mice was intermediate between non-Tg and homozygous Tg animals. This interpretation is supported by the observation that a similar phenotype was found in a second Tg line, thus making it very unlikely that the phenotype resulted from disruption or alteration of unknown gene(s) at the genomic site of transgene integration. More importantly, our data on the GPx-specific inhibitor MS confirmed that the synaptic transmission phenotype of these Tg models derived from increased GPx activity in the hippocampus.
A number of factors may contribute to the loss of synaptic transmission recovery after transient hypoxia. For instance, several studies have implicated the entry of calcium into glutamatergic neurons as a critical step responsible for hypoxia-induced neuronal injury (32, 33). An important route of calcium influx into pyramidal neurons in the CA1 region of the hippocampus is through activation of the NMDA subtype of glutamate receptors. Accordingly, activation of this receptor complex was shown to be associated with ischemia/reperfusion-induced cell damage (34) and generation of free radicals (31). Therefore, any significant difference in calcium influx into CA1 pyramidal neurons through NMDA receptor channels, or an alteration in calcium homeostasis, could have an impact on synaptic transmission recovery after hypoxia. However, a decrease of NMDA receptor function in the Tg-GPx mice investigated seems unlikely, on the basis of our data, showing that hippocampal slices from non-Tg and Tg mice (no hypoxic exposure) produced a similar level of LTP. Of course, as impairment of LTP formation after hypoxic insult was considerably reduced in the Tg hippocampus, our results suggest that the level of GPx activity may be an important protective factor contributing to the minimization of learning and memory deficits caused by hypoxic episodes.
In an attempt to further investigate the involvement of oxygen free radicals in the Tg synaptic transmission phenotypes, we determined carbonyl contents and TBARS formation of hippocampal slices as indices of free radical generation and oxidative damages of both protein and lipid components. Although we found that in Tg mouse hippocampal slices, protein and lipid oxidation capacities were significantly reduced in response to NMDA application, no substantial changes of oxidative stress could be detected in the hippocampal tissues after hypoxia. This result is not too surprising, given the previous report demonstrating that short hypoxic episodes are not capable of generating discernable oxidative damages in hippocampal slices (7). It remains possible that impairment of synaptic transmission by transient hypoxia could involve subtle redox alterations resulting from a transient rise in peroxides (a source of free radicals). This putative rise in peroxides, yet to be demonstrated, would not be sufficient for detection at the level of lipid peroxidation or carbonyl formation. Despite that, our NMDA data clearly support the hypothesis that recovery of synaptic transmission in Tg-GPx hippocampus after hypoxia may indeed be related to improved detoxification of peroxides that could be produced in response to NMDA-receptor activation. The target cellular mechanisms protected in the synaptic transmission and the LTP signaling pathways and machinery remain to be identified. Candidate mechanisms may include prevention of signaling interference involving kinase activation (or tyrosine phosphatase inhibition by free radicals) that may culminate, for example, in the inhibition of both IκB phosphorylation and NFκB activation (35); inhibition of peroxide-mediated activation of phospholipase A2 (36); and prevention of oxygen free radical-mediated damage to glutamate-uptake systems (37). Hypoxic insults were reported to transiently augment the extracellular concentration of glutamate (38). It will thus be of interest to find out to what extent the protective effects of GPx observed in this study are exerted on glutamate carriers by preventing free radical-mediated impairment of glutamate transport.
The GPx-mediated protective effects we have observed in vitro at the level of hippocampal function in Tg-GPx hippocampal slices exposed to hypoxic insults are in good agreement with the partial protection seen in vivo at the histochemical level in different GPx Tg mice after focal cerebral ischemia/reperfusion, as recently reported by Weisbrot-Lefkowitz et al. (12). A decrease in peroxide production associated with GPx overexpression had been demonstrated in these Tg mice (13), thus lending strong support for a major role of peroxide formation in cellular injury mediated by transient ischemia. It should be noted, however, that GPx overexpression in these mice may be detrimental to various physiological processes, including the ability to induce heat shock proteins in response to elevated temperature (13). It will therefore be of interest to compare the responses of our Tg-GPx mice to focal cerebral ischemia, because these animals seem to express lower levels of human GPx. Although both Tg-GPx lines investigated in this study showed a significant increase in GPx-like antigen in CA1 and CA3 pyramidal cells of the hippocampus, we do not know exactly to what extent GPx activity was augmented in immunoreactive cells. In addition, it remains to be determined how GPx immunoreactivity is distributed among the various cell types (neuronal and nonneuronal) of the brain.
Nevertheless, our results support the hypothesis that peroxide formation contributes to brain damage and synaptic dysfunction induced by transient hypoxia. Of course, it will be of particular interest to identify the mechanisms conferring LTP resistance to transient hypoxia, and possibly ischemia, in association with increased GPx activity.
Acknowledgments
This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada to G.M., grants from the National Cancer Institute and the Alzheimer's Association (Chicago) to M.-E.M., and a grant from the Réseau du Fond de la Recherche en Santé du Québec to G.M. and M.-E.M.
Abbreviations
- ACSF
artificial cerebrospinal fluid
- EPSP
excitatory postsynaptic potential
- GPx1
seleno glutathione peroxidase-1
- LTP
long-term potentiation
- MS
mercaptosuccinate
- MT
metallothionein
- NMDA
N-methyl-d-aspartate
- SOD
superoxide dismutase
- TBS
theta burst stimulation
- Tg
transgenic
- TBARS
thiobarbituric acid reactive substances
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.060574597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.060574597
References
- 1.Chan P H. Stroke. 1996;6:1124–1129. doi: 10.1161/01.str.27.6.1124. [DOI] [PubMed] [Google Scholar]
- 2.Samdani A F, Dawson T M, Dawson V L. Stroke. 1997;28:1283–1288. doi: 10.1161/01.str.28.6.1283. [DOI] [PubMed] [Google Scholar]
- 3.Sies H. Eur J Biochem. 1993;215:213–219. doi: 10.1111/j.1432-1033.1993.tb18025.x. [DOI] [PubMed] [Google Scholar]
- 4.Kinouchi H, Epstein C J, Mizui T, Carlson E, Chen S F, Chan P H. Proc Nat Acad Sci USA. 1991;88:11158–11162. doi: 10.1073/pnas.88.24.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang G, Chan P H, Chen J, Carlson E, Chen S F, Weinstein P, Epstein C J, Kamii H. Stroke. 1994;25:165–170. doi: 10.1161/01.str.25.1.165. [DOI] [PubMed] [Google Scholar]
- 6.Chan P H, Kamii H, Yang G, Gafni J, Epstein C J, Carlson E, Reola L. Neuroreport. 1993;5:4959–4965. doi: 10.1097/00001756-199312000-00028. [DOI] [PubMed] [Google Scholar]
- 7.Musleh W, Bruce A, Malfroy B, Baudry M. Neuropharmacology. 1994;33:929–934. doi: 10.1016/0028-3908(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 8.Mirault M E, Tremblay A, Beaudoin M, Tremblay M. J Biol Chem. 1991;266:20752–20760. [PubMed] [Google Scholar]
- 9.Mirault M E, Tremblay A, Furling D, Trépanier G, Dugré F, Puymirat J, Pothier F. Ann NY Acad Sci. 1994;738:104–115. doi: 10.1111/j.1749-6632.1994.tb21795.x. [DOI] [PubMed] [Google Scholar]
- 10.Dawson D A, Massayasu H, Graham D I, Macrae I M. Neurosci Lett. 1995;185:65–69. doi: 10.1016/0304-3940(94)11226-9. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida T, Watanabe M, Engelman D T, Engelman R M, Schley J A, Maulik N, Ho Y S, Oberley T D, Das D K. J Mol Cell Cardiol. 1996;28:1759–1767. doi: 10.1006/jmcc.1996.0165. [DOI] [PubMed] [Google Scholar]
- 12.Weisbrot-Lefkowitz M, Reuhl K, Perry B, Chan P H, Inouye M, Mirochnitchenko O. Mol Brain Res. 1998;53:334–339. doi: 10.1016/s0169-328x(97)00313-6. [DOI] [PubMed] [Google Scholar]
- 13.Mirochnitchenko O, Palnitkar U, Philbert M, Inouye M. Cell Biol. 1995;92:8120–8124. doi: 10.1073/pnas.92.18.8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherubini E, Ben-Ari Y, Krnjevic K. J Neurophysiol. 1989;62:882–894. doi: 10.1152/jn.1989.62.4.882. [DOI] [PubMed] [Google Scholar]
- 15.Arai A, Larson J, Lynch G. Brain Res. 1990;511:353–357. doi: 10.1016/0006-8993(90)90184-d. [DOI] [PubMed] [Google Scholar]
- 16.Stevens C F. Cell. 1996;87:1147–1148. doi: 10.1016/s0092-8674(00)81808-5. [DOI] [PubMed] [Google Scholar]
- 17.Trépanier G, Furling D, Puymirat J, Mirault M E. Neuroscience. 1996;75:231–243. doi: 10.1016/0306-4522(96)00222-9. [DOI] [PubMed] [Google Scholar]
- 18.Lavoie L, Tremblay A, Mirault M E. J Biol Chem. 1992;267:3632–3636. [PubMed] [Google Scholar]
- 19.Lee K, Oliver M, Schottler F, Lynch G. In: Electrical Activity in Isolated Mammalian CNS Preparations. Kerkut G, Wheal H V, editors. New York: Academic; 1981. pp. 189–212. [Google Scholar]
- 20.Barrier L, Page G, Fauconneau B, Juin F, Tallineau C. Free Radical Res. 1998;28:411–422. doi: 10.3109/10715769809070810. [DOI] [PubMed] [Google Scholar]
- 21.Levine R L, Williams J A, Stadtman E R, Shacter E. Methods Enzymol. 1994;233:346–357. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- 22.Arai A, Kessler M, Lee K, Lynch G. Brain Res. 1990;532:63–68. doi: 10.1016/0006-8993(90)91742-y. [DOI] [PubMed] [Google Scholar]
- 23.Chaudiere J, Wilhelmsen E C, Tappel A L. J Biol Chem. 1984;259:1043–1050. [PubMed] [Google Scholar]
- 24.Dugan L L, Sensi S L, Canzoniero L M, Handran S D, Rothman S M, Lin T S, Goldberg M P, Choi D W. J Neurosci. 1995;15:6377–6388. doi: 10.1523/JNEUROSCI.15-10-06377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Budd S L. Pharmacol Ther. 1998;80:203–229. doi: 10.1016/s0163-7258(98)00029-1. [DOI] [PubMed] [Google Scholar]
- 26.Mattson M P. Neurosci Behav Rev. 1997;21:193–206. doi: 10.1016/s0149-7634(96)00010-3. [DOI] [PubMed] [Google Scholar]
- 27.Charriaut-Marlangue C, Pollard H, Kadri-Hassani N, Khrestchatisky M, Moreau J, Dessi F, Kang K I, Ben-Ari Y. Eur J Neurosci. 1992;4:766–776. doi: 10.1111/j.1460-9568.1992.tb00186.x. [DOI] [PubMed] [Google Scholar]
- 28.Choi D W. J Neurosci. 1990;10:2493–2501. doi: 10.1523/JNEUROSCI.10-08-02493.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gage A T, Stanton P K. Brain Res. 1996;719:172–178. doi: 10.1016/0006-8993(96)00092-3. [DOI] [PubMed] [Google Scholar]
- 30.Bromont C, Marie C, Bralet J. Stroke. 1989;20:918–924. doi: 10.1161/01.str.20.7.918. [DOI] [PubMed] [Google Scholar]
- 31.Lafon-Cazal M, Pietri S, Culcasi M, Bockaert J. Nature (London) 1993;364:535–537. doi: 10.1038/364535a0. [DOI] [PubMed] [Google Scholar]
- 32.Siesjoe B K. Ann NY Acad Sci. 1988;522:638–661. doi: 10.1111/j.1749-6632.1988.tb33410.x. [DOI] [PubMed] [Google Scholar]
- 33.Siesjoe B K, Smith M L. Arzneim-Forsch. 1991;41:288–292. [PubMed] [Google Scholar]
- 34.Simon R P, Swan J H, Griffiths T, Meldrum B S. Science. 1984;226:850–852. doi: 10.1126/science.6093256. [DOI] [PubMed] [Google Scholar]
- 35.Kretz-Remy C, Mehlen P, Mirault M E, Arrigo A P. J Cell Biol. 1996;133:1083–1093. doi: 10.1083/jcb.133.5.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chakraborti S, Chakraborti T. Cell Signalling. 1995;7:75–83. doi: 10.1016/0898-6568(94)00061-f. [DOI] [PubMed] [Google Scholar]
- 37.Berman S B, Hastings T G. J Neurochem. 1997;69:1185–1195. doi: 10.1046/j.1471-4159.1997.69031185.x. [DOI] [PubMed] [Google Scholar]
- 38.Ghribi O, Callebert J, Plotkine M, Boulu R G. Neurosci Lett. 1994;174:34–38. doi: 10.1016/0304-3940(94)90112-0. [DOI] [PubMed] [Google Scholar]