Abstract
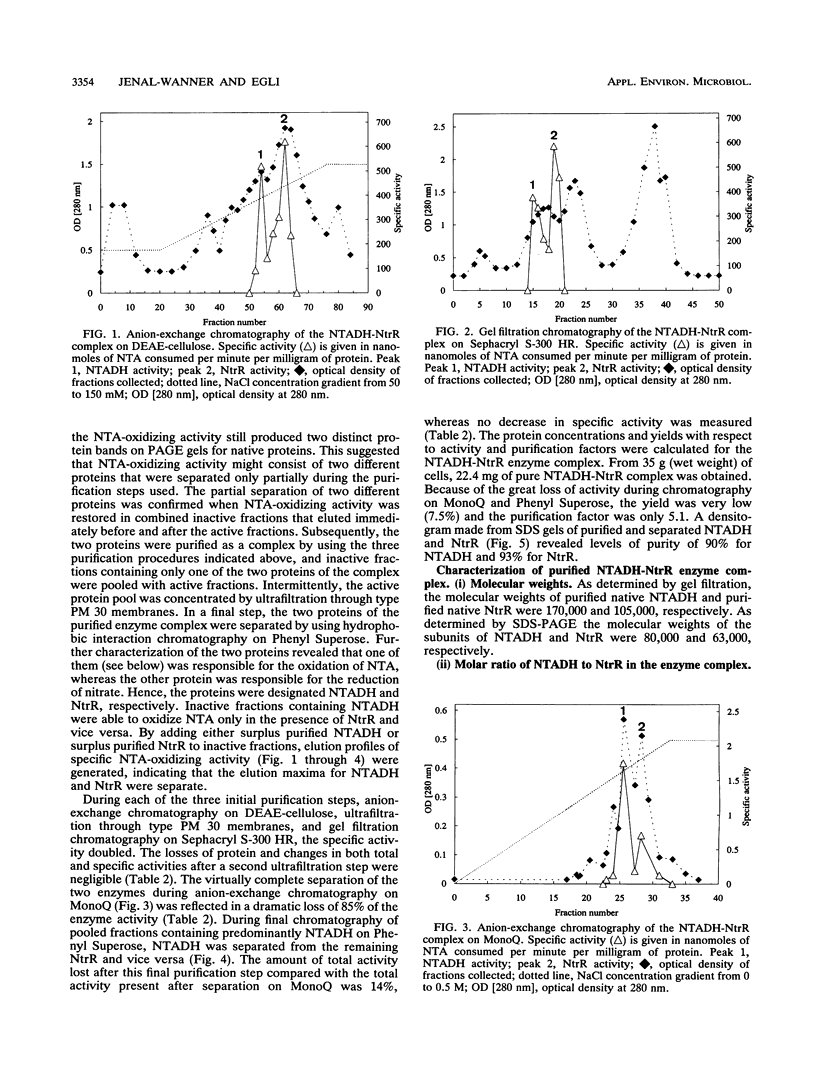
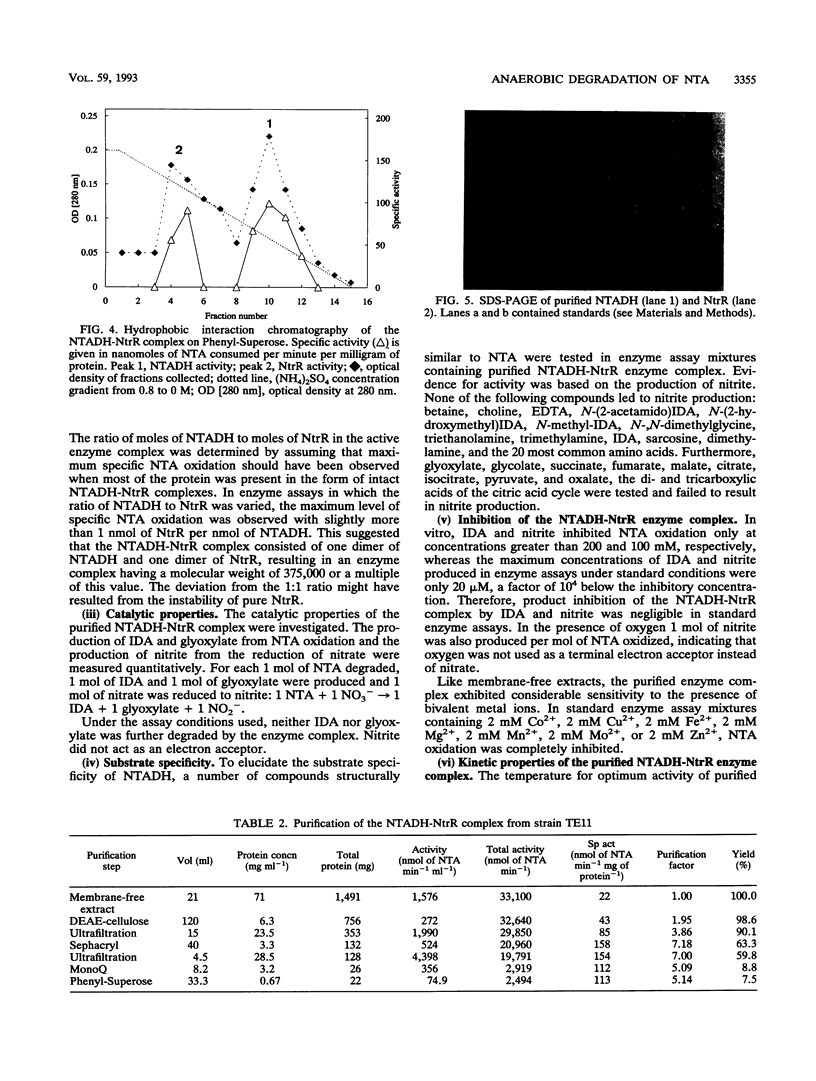
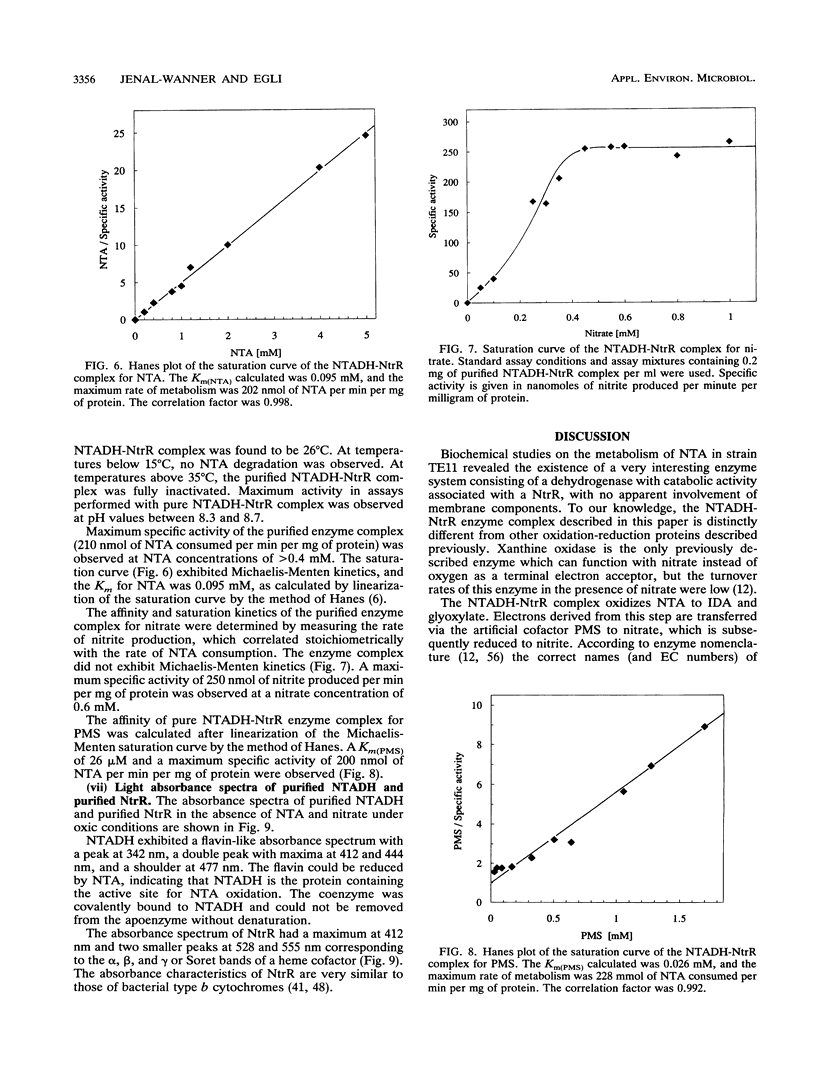
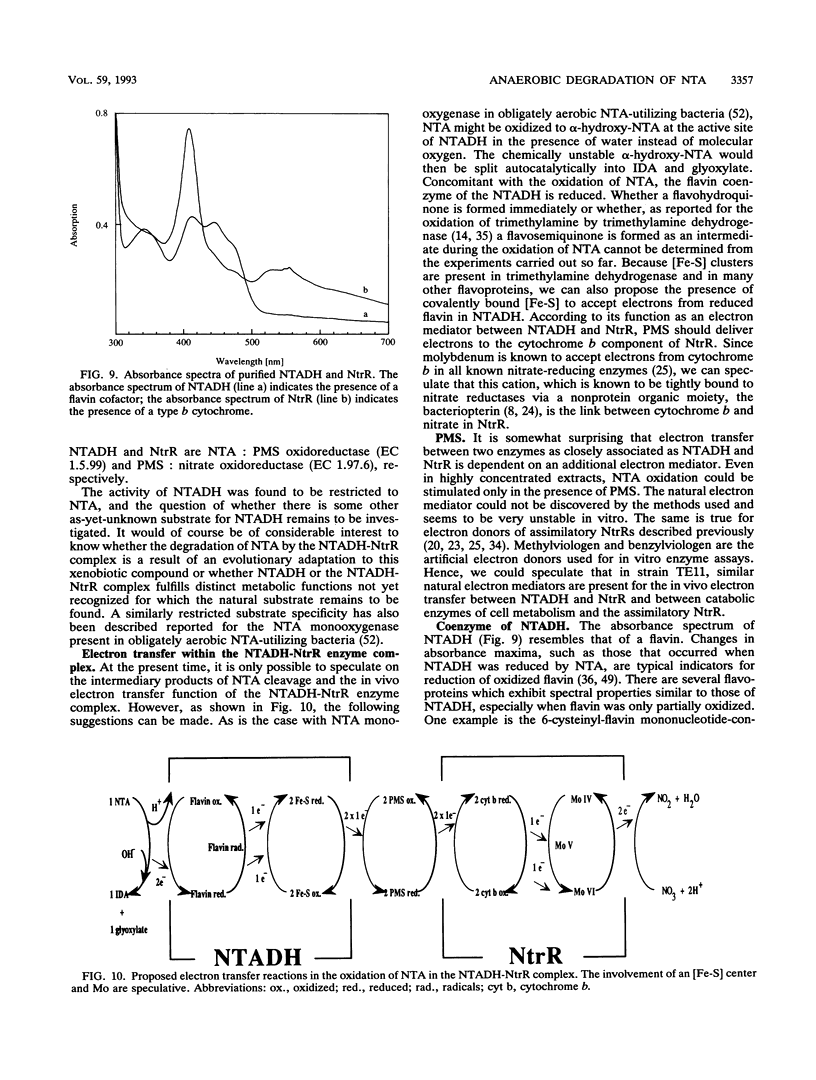
The initial step in the anoxic metabolism of nitrilotriacetate (NTA) was investigated in a denitrifying member of the gamma subgroup of the Proteobacteria. In membrane-free cell extracts, the first step of NTA oxidation was catalyzed by a protein complex consisting of two enzymes, NTA dehydrogenase (NTADH) and nitrate reductase (NtR). The products formed were iminodiacetate and glyoxylate. Electrons derived from the oxidation of NTA were transferred to nitrate only via the artificial dye phenazine methosulfate, and nitrate was stoichiometrically reduced to nitrite. NTADH activity could be measured only in the presence of NtrR and vice versa. The NTADH-NtrR enzyme complex was purified and characterized. NTADH and NtrR were both alpha 2 dimers and had molecular weights of 170,000 and 105,000, respectively. NTADH contained covalently bound flavin cofactor, and NtrR contained a type b cytochrome. Optimum NTA-oxidizing activity was achieved at a molar ratio of NTADH to NtrR of approximately 1:1. So far, NTA is the only known substrate for NTADH. This is the first report of a redox enzyme complex catalyzing the oxidation of a substrate and concomitantly reducing nitrate.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. L., Bishop W. E., Campbell R. L. A review of the environmental and mammalian toxicology of nitrilotriacetic acid. Crit Rev Toxicol. 1985;15(1):1–102. doi: 10.3109/10408448509023766. [DOI] [PubMed] [Google Scholar]
- Anraku Y. Bacterial electron transport chains. Annu Rev Biochem. 1988;57:101–132. doi: 10.1146/annurev.bi.57.070188.000533. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bray R. C. The reactions and the structures of molybdenum centers in enzymes. Adv Enzymol Relat Areas Mol Biol. 1980;51:107–165. doi: 10.1002/9780470122969.ch3. [DOI] [PubMed] [Google Scholar]
- Cripps R. E., Noble A. S. The metabolism of nitrilotriacetate by a pseudomonad. Biochem J. 1973 Dec;136(4):1059–1068. doi: 10.1042/bj1361059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D. E., Tollin G. Semiquinone formation in flavo- and metalloflavoproteins. Top Curr Chem. 1983;108:109–138. doi: 10.1007/3-540-11846-2_4. [DOI] [PubMed] [Google Scholar]
- Ghisla S., Massey V. Studies on the catalytic mechanism of lactate oxidase. Formation of enantiomeric flavin-N(5)-glycollyl adducts via carbanion intermediates. J Biol Chem. 1980 Jun 25;255(12):5688–5696. [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Hinton S. M., Dean D. Biogenesis of molybdenum cofactors. Crit Rev Microbiol. 1990;17(3):169–188. doi: 10.3109/10408419009105724. [DOI] [PubMed] [Google Scholar]
- Hochstein L. I., Tomlinson G. A. The enzymes associated with denitrification. Annu Rev Microbiol. 1988;42:231–261. doi: 10.1146/annurev.mi.42.100188.001311. [DOI] [PubMed] [Google Scholar]
- Ingledew W. J., Poole R. K. The respiratory chains of Escherichia coli. Microbiol Rev. 1984 Sep;48(3):222–271. doi: 10.1128/mr.48.3.222-271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S. A. NTA removal in septic tank and oxidation pond systems. J Water Pollut Control Fed. 1974 Jan;46(1):78–88. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Müller F. Flavin radicals: chemistry and biochemistry. Free Radic Biol Med. 1987;3(3):215–230. doi: 10.1016/0891-5849(87)90009-8. [DOI] [PubMed] [Google Scholar]
- Pichinoty F., Azoulay E., Couchoud-Beaumont P., Le Minor L., Rigano C., Bigliardi-Rouvier J., Piéchaud M. Recherche des nitrate-réductases bactériennes A et B: résultats. Ann Inst Pasteur (Paris) 1969 Jan;116(1):27–42. [PubMed] [Google Scholar]
- Poole R. K. Bacterial cytochrome oxidases. A structurally and functionally diverse group of electron-transfer proteins. Biochim Biophys Acta. 1983 Sep 15;726(3):205–243. doi: 10.1016/0304-4173(83)90006-x. [DOI] [PubMed] [Google Scholar]
- Schneider R. P., Zürcher F., Egli T., Hamer G. Determination of nitrilotriacetate in biological matrices using ion exclusion chromatography. Anal Biochem. 1988 Sep;173(2):278–284. doi: 10.1016/0003-2697(88)90190-x. [DOI] [PubMed] [Google Scholar]
- Sias S. R., Stouthamer A. H., Ingraham J. L. The assimilatory and dissimilatory nitrate reductases of Pseudomonas aeruginosa are encoded by different genes. J Gen Microbiol. 1980 May;118(1):229–234. doi: 10.1099/00221287-118-1-229. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Davis P. S., Kamin H. Reduced nicotinamide adenine dinucleotide phosphate-sulfite reductase of enterobacteria. 3. The Escherichia coli hemoflavoprotein: catalytic parameters and the sequence of electron flow. J Biol Chem. 1974 Mar 10;249(5):1572–1586. [PubMed] [Google Scholar]
- Smith L. Bacterial cytochromes and their spectral characterization. Methods Enzymol. 1978;53:202–212. doi: 10.1016/s0076-6879(78)53025-5. [DOI] [PubMed] [Google Scholar]
- Steenkamp D. J., Beinert H. Mechanistic studies on the dehydrogenases of methylotrophic bacteria. 2. Kinetic studies on the intramolecular electron transfer in trimethylamine and dimethylamine dehydrogenase. Biochem J. 1982 Nov 1;207(2):241–252. doi: 10.1042/bj2070241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trijbels F., Vogels G. D. Degradation of allantoin by Pseudomonas acidovorans. Biochim Biophys Acta. 1966 Feb 14;113(2):292–301. doi: 10.1016/s0926-6593(66)80068-1. [DOI] [PubMed] [Google Scholar]
- Uetz T., Schneider R., Snozzi M., Egli T. Purification and characterization of a two-component monooxygenase that hydroxylates nitrilotriacetate from "Chelatobacter" strain ATCC 29600. J Bacteriol. 1992 Feb;174(4):1179–1188. doi: 10.1128/jb.174.4.1179-1188.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner U., Kemmler J., Weilenmann H. U., Egli T., el-Banna T., Auling G. Isolation and growth of a bacterium able to degrade nitrilotriacetic acid under denitrifying conditions. Biodegradation. 1990;1(1):31–41. doi: 10.1007/BF00117049. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



