Abstract
L-type voltage-sensitive Ca2+ channels (L-VSCCs) play an important role in developmental and aging processes, as well as during normal function of brain neurons. Here, we tested a prediction of the hypothesis that membrane density of functional L-VSCCs is regulated by the level of gene expression for its α1D pore-forming subunit. If so, α1D mRNA and L-VSCC activity should be positively correlated within individual neurons. Conventional methods of aspiration and/or acute cell dissociation used in prior single-cell studies have generally yielded variable and incomplete recovery of intracellular mRNA. Thus, quantitative relationships between channel function and expression have been difficult to define. In this study, we used the partially dissociated (“zipper”) hippocampal slice preparation as a method for collecting a single neuron's mRNA complement. This preparation, developed to expose neuronal somata for recording, also enables the extraction of a neuron with major processes largely intact. Thus, single-cell measures of gene/mRNA expression can be based on approximately the cell's full set of mRNA transcripts. In adult and aged rat hippocampal zipper slices, L-VSCC activity was first recorded in CA1 neurons in cell-attached patch mode. The same neurons were then extracted and collected for semiquantitative reverse transcriptase–PCR analysis of α1D and calmodulin A (CaM) mRNA content. Across multiple single neurons, a significant, positive correlation was found between the rank orders of L-VSCC activity and of α1D, but not CaM, mRNA expression. Thus, these studies support the possibility that the level of α1D gene expression regulates the density of functional L-VSCCs.
L-type voltage-sensitive Ca2+ channels (L-VSCCs) are distributed broadly in the central nervous system and are important to a wide range of neuronal functions (1, 2), including developmental regulation (3–6), gene expression (7, 8), control of excitability and dendritic function (9–11), learning and memory (12, 13), and long-term potentiation (14, 15) or depression (16). Altered functions of L-type VSCCs are also implicated in pathologic conditions, such as excitotoxicity (17), diabetes (18–21), and amyotrophic lateral sclerosis (22–24).
In addition, substantial evidence points to the importance of alterations in L-VSCCs in brain aging. Several Ca2+-dependent potentials and Ca2+ currents mediated by L-VSCCs are increased in hippocampal neurons of aged rats (25–28) and rabbits (9, 13), as are L-VSCC-mediated LTP or LTD (15, 16). Similar increases in L-VSCC currents occur in hippocampal neurons with age in culture (29, 30). Studies at the single-channel level indicate that many of these aging changes in Ca2+ currents may depend on an increase in the membrane density of functionally available L-VSCCs (29, 31).
Yet, it remains unclear how neuronal L-VSCC density is regulated on a continuing basis. One possibility is that the level of gene expression for one or more L-VSCC subunits plays a major role. However, increased L-VSCC density (29, 31) is not sufficient by itself to infer new synthesis because the functional availability of ion channels can also be regulated by other factors (e.g., phosphorylation, anchoring). Nevertheless, other evidence is at least indirectly consistent with a gene expression hypothesis. VSCCs are composed of up to four distinct subunits (α1, β, α2-δ, γ) (32), and two α1 subunit genes have been identified that encode for L-VSCCs in the brain (α1D and α1C) (33–35). In several studies in which functional L-VSCCs were found to be elevated with aging in hippocampal neurons, ribonuclease protection assays or in situ hybridization methods showed that α1D mRNA was also increased, whereas β1 subunit mRNA was stable (29, 36). One major prediction of a gene expression hypothesis is that mRNA of a subunit (e.g., α1D) should be correlated quantitatively with L-VSCC activity under various conditions. However, studies of mean values in a few age groups, such as those above, usually cannot provide sufficient degrees of freedom to support a strong statistical inference of correlation. Further, cell type diversity and the inability to record from the same cells in which mRNA is measured both contribute error that impedes estimates of degree of association.
The problems of cell diversity have led a number of investigators to develop methods for performing electrophysiology and mRNA amplification/measurement in the same neuron (37–42). In most of these single-cell studies, the electrophysiology of the cell has been assessed by whole-cell patch clamp, and the intracellular contents have then been aspirated into the recording pipette (but see ref. 43). In several of these studies, adult neurons have been studied after acute dissociation (44). After reverse transcription (RT) of the cell's mRNA, cDNA has been amplified either by linear replication (39) or PCR (37, 38). These methods typically result in membrane rupture, process amputation, and collection of a highly variable fraction of the cell's mRNA transcripts. Consequently, semiquantitative measures on the aspirated fraction require the target mRNA to be normalized to another molecular species in the cell, which adds the error of the control species' measurement and regulation. Moreover, dendritic mRNA, which is increasingly recognized to serve selective functions (45), is lost. These factors have made quantitative estimates of expression in a single neuron difficult, and thus most prior experiments have focused on associations of all-or-none expression with function (but see refs. 43 and 46).
Clearly, there would be a major measurement advantage in single-neuron studies if approximately the full set of mRNA transcripts could be collected from each neuron. With such improved quantitation, single-cell studies, which minimize problems of cell diversity and inadequate degrees of freedom, could then be used more effectively to test predicted expression–function correlations.
In this study, therefore, we used the partially dissociated hippocampal slice preparation as a method for collecting nondisrupted neurons that contain largely complete sets of mRNA transcripts. This preparation (also termed the “zipper slice” for its tendency to gradually open along cell body layers) was originally developed by Gray, Johnston, and colleagues (47) in guinea pigs for the purpose of exposing cell bodies to patch pipettes with minimal trauma. Subsequently, we adapted the preparation for single-channel recording studies in aged rats (31). After recording, we are able to extract a largely intact neuron from the weakened connections of the zipper slice by maintaining negative pipette pressure and carefully withdrawing the pipette. This preparation was used here to test the α1D mRNA correlation with L-VSCC activity predicted by an α1D gene expression hypothesis of L-VSCC regulation.
Materials and Methods
Tissue Preparation.
Brown–Norway × Fischer 344 hybrid male rats (ages: 8–35 mo, n = 11) were anesthetized with CO2 gas and decapitated. Whole brains were removed and placed in ice-cold, oxygenated artificial cerebrospinal fluid (aCSF, in mM: 114 NaCl, 2.5 KCl, 2 CaCl2, 8 MgCl2, 30 NaHCO3, and 10 glucose) for ≈45 s. Methods used in the preparation of zipper slices were similar to those reported by Gray et al. (47), as adapted for aged and adult rat neurons (31). The chilled brain was removed, and the hippocampi were quickly dissected out, sectioned in 350-μm-thick slices on a tissue chopper (Brinkmann), and immersed in oxygenated aCSF at 31.5°C in a covered, 35-mm plastic culture dish. Usually 12 slices, as well as the remaining, uncut hippocampal tissue, were placed in the culture dish. A stream of 95% O2/5% CO2 gas, prehumidified by bubbling through water, was directed at the surface of the slice-incubation media. Slices were treated with 0.7 mg/ml pronase for 30 min, followed by 0.5 mg/ml thermolysine for 15 min. Half of the thermolysine solution was removed afterward and replaced with oxygenated aCSF, effectively halving the concentration of thermolysine.
Thirty minutes after the last solution change, the first slice was removed from the incubator to begin the “unzipping” procedure. The incubation medium was replaced with an oxygenated “shaking” solution (as aCSF, except 2 mM CaCl2 was substituted with 2 mM EGTA). The slice was washed three times and left in shaking solution for 2 min, then taken out of solution, and the CA3 region was removed with a scalpel. The slice was then returned to shaking solution (≈1 ml) in a clear plastic tube sealed with Parafilm M and gently shaken by hand. Periodically (every 1–3 agitations), the tube was inspected for increases in the length of the hippocampal CA1 field dissociation (unzipping) along the stratum pyramidale, and for floating debris. Shaking solution was replaced periodically with fresh solution. Once the CA1 separation was clearly visible, the slice was placed in the recording perfusion chamber.
Cell Identification and L-type Ca2+ Channel Recording.
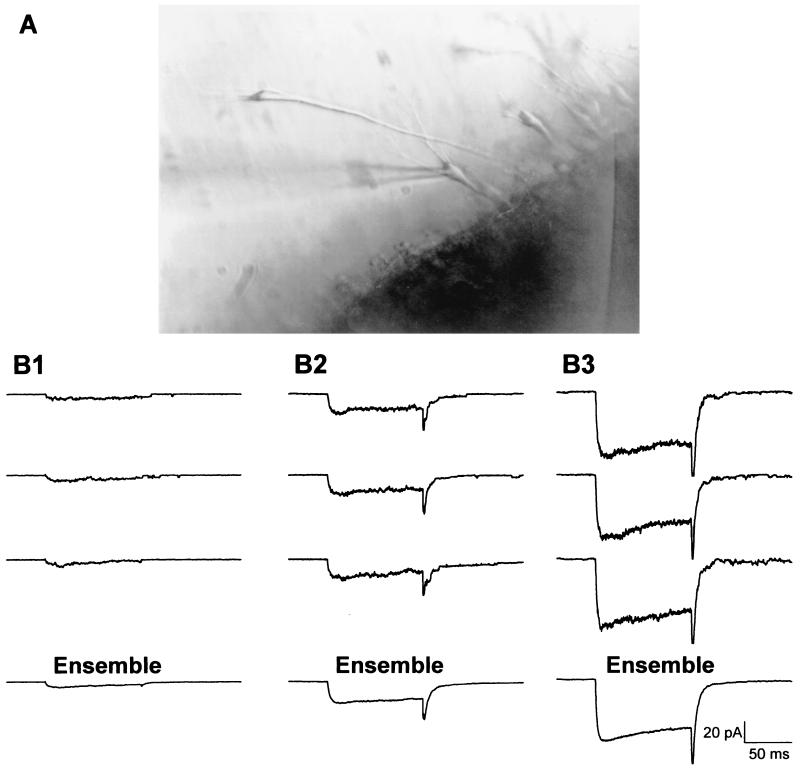
The bath recording solution (in mM: 140 K gluconate, 3 MgCl2, 10 glucose, 10 EGTA, and 10 Hepes, pH to 7.35 with KOH, osmolarity adjusted to 300 mOsm by dilution with distilled water) was rapidly perfused (8.5 ml/min) across the slice for 1 min to wash out shaking solution. During recording, the bath was static. The pipette solution contained, in mM: 20 BaCl2, 90 choline Cl, 10 TEA, and 10 Hepes; pH was adjusted to 7.35 by TEA OH, and osmolarity was adjusted to 290 mOsm with sucrose. Electrodes were pulled from capillary glass (1.6-mm outer diameter; Drummond Scientific, Broomall, PA) on a Flaming–Brown horizontal puller (Sutter Instruments, Novato, CA), coated with Sylgard (Dow-Corning), and fire polished on a microforge (Narishige, Tokyo) just before use. Tip resistance averaged 4.23 (±0.12) MΩ. With the slice in the perfusion chamber, pyramidally shaped neurons in field CA1 were identified under the microscope. Neurons that appeared to be swollen, rounded, or flattened were avoided. Cell bodies were unsupported in the medium and carefully approached with the pipette tip; when contact was made, a high-resistance seal was formed in cell-attached mode using standard protocols (48). Mean seal resistance was 24.7 (±2.6) GΩ. Voltage-clamp protocols that stepped the membrane from −70 to 0 mV for 150 ms were used to activate voltage-sensitive Ca2+ channels. Steps from −70 to −140 mV were used to obtain traces for subsequent offline subtraction of leak current. Fifteen consecutive sweeps from each patch (45-s interpulse interval) were used to construct an average ensemble. The total area within the ensemble average was integrated and divided by step duration to obtain mean patch current (Fig. 1).
Figure 1.
Recording from cells with intact neurites. (A) The recording electrode is patched onto a CA1 pyramidal neuron in a zipper slice. (B1–B3) Recorded multichannel patch VSCC current varies in amplitude among different neurons. The first three traces in each example are individual current records of multichannel patch activity in three representative neurons. The fourth trace (ensemble) is the average of 15 such individual records for each cell.
To facilitate the analysis of L-VSCCs, BayK 8644 (0.5 μM) was added to the recording pipette. When treated with BayK 8644 (see above), L-type channels exhibit a 10-fold increase in mean open time (49–51), and total patch current is dominated by L-type channel current (cf. 31; Fig. 1). Previous studies have shown that, under these conditions, ensemble averages closely reflect L-type VSCC density (29, 31). Total recording time was usually held to 15 min per patch. There was no apparent alteration in mRNA yield with this duration of recording, and the zipper preparation yielded healthy cells for ≈ 4 h, after which no cells were recorded or collected.
Cell Collection and RT.
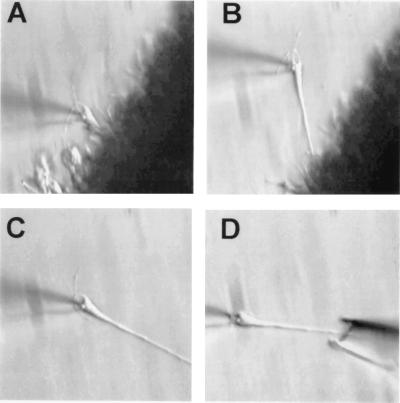
Following recording protocols, as described above, the same individual CA1 pyramidal neurons were collected for mRNA analysis. With the recording pipette still attached to the neuron, a slight negative pressure was applied, and the neuron was gently pulled away from the slice. Because of enzymatic loosening, the apical dendrite usually slides out readily (Fig. 2). Most of the basilar dendrites and the main apical dendritic tree appear intact, although the possibility that some small branches are amputated and remain in the slice has not yet been examined in detail. After extraction from the slice, each neuron was positioned in front of the inlet port of the perfusion chamber, and perfusion was initiated for ≈30 s. This procedure ensured that no tissue debris was collected. Multiple sham experiments, in which the surrounding bath solution was collected and processed in parallel as a negative control, were also conducted.
Figure 2.
A representative cell is pulled free of the slice and collected for molecular analysis. (A) A high-resistance seal is formed between the cell and the recording pipette to record VSCC activity. (B) The recording electrode is used to pull the cell free of the enzymatically treated slice. (C) Once removed from the slice, the cell, still attached to the recording pipette, is cleaned by perfusion with uncontaminated bath solution. (D) The cleaned cell is then collected with a larger pipette by capillary action.
Cell collection pipettes were prepared from the same Drummond glass used for recording electrodes. Capillary glass tubes were silinized (exposed to 1 ml volatile trimethylchlorosilane in a vacuum bell jar for 24 h) and baked at 180°C for 4 h. Treated capillary glass was then pulled in a horizontal puller, and the tips were broken back and fire polished to yield a ≈10-μm tip opening. Slight positive pressure was applied while lowering empty collector pipettes into the bath to prevent the incidental collection of unrelated material. This pressure was released as the collection pipette neared the target cell at the tip of the recording pipette. Capillary action was then used to draw the intact neuron from the recording electrode into the collection pipette (Fig. 2). To confirm that the neurons were largely intact at collection, this capture procedure was videotaped for each cell.
The collected cell was immediately transferred to a prechilled microcentrifuge tube containing RT solution: 1× PCR buffer (Perkin–Elmer), 3.75 mM MgCl2, 0.5 mM each of dATP, dGTP, dCTP, and dTTP; 0.25 μM of random hexamers, 1 unit/ml RNase inhibitor (Roche Molecular Biochemicals), 0.5 μg/μl gene 32 protein, and 2.5 units Moloney murine leukemia virus reverse transcriptase (Perkin–Elmer). For cDNA synthesis, each cell was incubated at 42°C for 1 h in a total volume of 20 μl RT solution, followed by 10 min at 95°C. The cDNA was stored at −80°C until used for PCR analysis.
Single-Cell PCR.
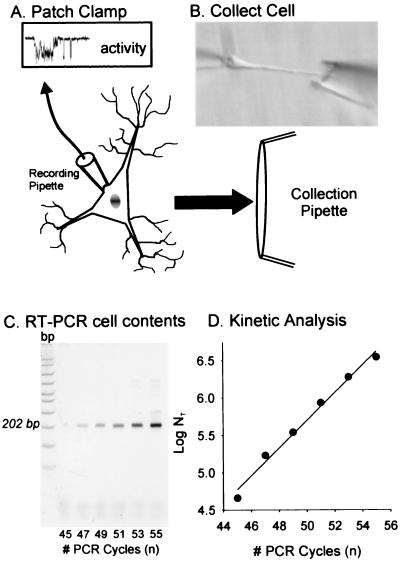
Because the L-type α1D subunit is a relatively rare message, we selected the RT-PCR (37) rather than the RT-linear (39) amplification method; the cDNA obtained from each individual cell is sufficient to provide a template for PCR amplification of several (2–5) mRNA species. Both the L-VSCC α1D subunit and calmodulin A (CaM) messages were analyzed from each neuron. To measure the amount of α1D message in a cell, two rounds of PCR amplification were required using primers specific for the α1D cDNA sequence. The sense and antisense primers used for α1D were: sense (5′-GTTACTATTGATGACTATCA-3′) and antisense (5′-CTAAGAATGAAGAATGCGCT-3′), which span a II-III linker region of the α1D sequence (nucleotides 2930–3132, GenBank M57682, rat brain) and generate a 202-bp PCR fragment. The first round of PCR was performed in a 10-μl reaction mixture (1× PCR buffer, 3.0 mM MgCl2, 0.4 mM dNTPs, 0.2 μM each of the primers, and 0.25 units AmpliTaq Gold DNA polymerase), to which 1/5 of a single cell's RT cDNA was added as template for amplification. The PCR protocol was as follows: 94°C for 3 min followed by 25 cycles at 94°C for 45 s, 50°C for 45 s, and 72°C for 1 min, and a final extension incubation at 72°C for 7 min. At the end of this first round of PCR, 90 μl of PCR mixture was added (total 100 μl) and a second round of PCR was carried out for 30 cycles. During the second PCR round, aliquots were collected from cycle 18 to cycle 28 at two-cycle intervals to monitor the amplification progress. The PCR products in the aliquots were analyzed on 9% polyacrylamide gels, stained with SYBRGold (Molecular Probes), and quantified on a Storm PhosphorImager (Molecular Dynamics). For the CaM message, only one round of PCR was required with 1/10 of single-cell RT cDNA added to a 100-μl reaction mixture. PCR was performed through 45 cycles (94°C for 45 s, 60°C for 45 s, and 72°C for 1 min). Aliquots were collected from cycles 40–45, and the PCR products were analyzed and quantitated. The sense and antisense primers for CaM were: sense (5′-GGTTGTCTGTTCTGGTCT-3′) and antisense (5′-AGGGAAGTCGATTGTGCCATTA-3′), which span a noncoding region at the 5′ end of the CaM sequence (nucleotides 30–272, GenBank M17069, rat brain), and yield a single 252-bp DNA fragment. Both the α1D and CaM PCR fragments were gel purified and confirmed by DNA sequencing. The procedural steps are summarized in Fig. 3. Note that the exponential phase of the reaction was defined on a log-linear kinetic plot for each cell, and one cycle was selected for comparison across cells.
Figure 3.
Summary of the steps for collection and molecular analysis of a recorded cell. (A) Neurons are exposed and recorded from enzymatically treated slices (see Materials and Methods). (B) Neurons are pulled free of the tissue, washed, collected, and transferred to an RT solution. The collected material is subdivided into aliquots, each of which can be examined for a different message. (C) A representative gel shows the amount of RT-PCR signal generated at different cycle numbers for the VSCC α1D message. (D) The signal intensity from each cycle (C) can then be used to generate a kinetic curve.
Data Analysis.
The efficiency of a PCR determines the quantity of PCR product at a given cycle, according to the equation: Log NT = n Log(1 + e) + LogNO, where NT is total PCR product at n cycle, NO is the starting mRNA amount, and e is the amplification efficiency. The efficiency is given by slope of the line in the linear phase of the log-linear plot (52). Cycle 45 of the PCR reaction was chosen for α1D analysis because it showed good signal strength, but was still well within the exponentially rising phase (i.e., nonsaturated) of the amplification process for all cells. The PCR products from the 45th cycles for all cells were run on the same acrylamide gel in separate lanes. This eliminated intergel variability, and allowed direct comparisons of relative signal strength among different cells. The same procedure was used for CaM analysis, except cycle 40 was chosen. Because of the relatively high number of PCR cycles used in the present study, negative controls containing no RT-PCR product were also run for the same number of cycles. In no case did a negative control generate either an α1D or a CaM signal. The current amplitude (pA) and mRNA values were not normally distributed, and therefore, were rank ordered, and the correlations across cells were tested by a nonparametric method (Spearman's test; ref. 53).
Results
Single-Channel Recording.
After mild enzymatic treatment and unzipping, a total of 31 pyramidal CA1 neurons from the hippocampal zipper slices of 7 young adult rats (7–8 mo) and 4 aged rats (33–35 mo) were visually identified and recorded in cell-attached mode for single channel L-VSCC activity (Fig. 1). However, approximately half of the cells exhibited no sign of channel activity or did not remain viable throughout the full recording protocol. Of the 14 cells that were both recorded and collected, 3 did not show a detectable α1D mRNA signal. Because only cells showing both electrophysiologic activity and mRNA signal were used, the final sample size was 11 (n = 8 young cells, 3 aged cells). All showed comparable amplification efficiencies. The cells that did not meet these criteria were probably damaged in preparation, although the possibility that some cells do not express L-VSCC or express only α1C cannot be ruled out.
The multichannel patch current amplitude varied substantially (3–100 pA; Fig. 1), and was not correlated with electrode-tip resistance within the study, indicating that differences in current amplitude were probably not because of variation in patch size (54). In addition, the mean amplitude of the multichannel patch current here was greater than that reported from previous preparations using similar techniques (31), which possibly reflects rat strain differences or subtle differences in preparation or techniques. Mean current was 17.9 (±7.0) pA for all 11 neurons.
PCR Quantitation of α1D mRNA in Single Neurons.
Eleven cells that showed comparable amplification efficiencies with no saturation (see Materials and Methods) were compared at the same PCR cycle number on the same gel. Therefore, RT-PCR product should be proportional to initial α1D transcript level in each cell. CaM mRNA levels in eight of the same neurons met similar criteria and were also studied for correlation with L-VSCC activity.
Rank-Order Correlation of α1D mRNA with L-VSCC Activity.
Cells were ranked on both variables and tested by the Spearman Rank Order Correlation test (53). The relative α1D levels estimated by single-cell RT-PCR showed a significant positive correlation with the rank order of mean currents in the same cells (rs = 0.64, P < 0.05; Fig. 4). However, no correlation was found between CaM message levels and mean currents of L-type Ca2+ channel activity within the same cells (Fig. 4).
Figure 4.
Correlation between channel activity and message level was specific for α1D mRNA. (A) Relationship between L-type Ca2+ channel activity and L-type α1D subunit mRNA level (estimated by RT-PCR) across individual neurons. A significant positive correlation (rs = 0.64, P < 0.05, Spearman's nonparametric test) was found between rank orders of L-VSCC activity and α1D mRNA level across individual cells. On the vertical axis, cells were ranked according to mRNA level. On the horizontal axis, the same cells were ranked according to channel activity. (B) No significant correlation (rs = 0.07; n.s.; Spearman's nonparametric test) was found between calmodulin (CaM) mRNA level and L-type Ca2+ channel activity in a subset of the same cells as A.
Discussion
The primary finding of this study is that the content of α1D mRNA showed a significant, positive rank-order correlation with L-VSCC activity among aged and adult rat CA1 neurons. A similar correlation was not found for CaM mRNA. Approximately 40% of the variance of relative L-VSCC activity, therefore, may be accounted for by variation in α1D gene expression.
The functional criteria used in these initial experiments restricted the cell yield, and consequently did not permit a statistical comparison between age groups. Nevertheless, the combined sample size of all cells was sufficient to detect a significant correlation across individual cells. Furthermore, we have recently replicated the correlation of α1D mRNA and L-VSCC activity in single CA1 neurons of young adult rats (ref. 55, and K.C.C. and E.M.B., unpublished observations). Therefore, whereas these present studies do not directly address aging differences, they support an important prediction of the hypothesis that α1D gene expression in part regulates functional L-VSCC density. Consequently, they considerably strengthen the possibility that previously observed age-dependent alterations in gene expression (36) fully or partly underlie the increased density of L-VSCCs in aged neurons (31).
Although mRNA setpoint levels are more commonly regulated by gene expression than by control of mRNA decay, further studies will be needed to examine a possible role for mRNA decay in regulation. Additional studies will also be needed to determine whether there is a partial contribution to L-VSCC variation from other subunit genes (e.g., α1C, which is found in many of the same neurons; ref. 56) or from posttranslational factors such as phosphorylation.
Nevertheless, the data suggest that the relationship of α1D mRNA to L-VSCC is in part accounted for by α1D gene expression levels. If so, the present results also appear to have implications for the kinetic pattern of genomic regulation of Ca2+ channel function. Subunit gene expression could conceivably operate through a burst of activity to establish a set level of protein and then remain quiescent until activated again to compensate for channel protein decay. An alternative pattern, which seems more probable if protein turnover is comparatively rapid, is one in which mRNA expression and protein turnover are in equilibrium and the level of mRNA synthesis can regulate the level of protein availability within relatively short periods. The latter mode appears to predict a consistent, quantitative correlation between α1D mRNA and L-VSCC activity more than the former.
Thus, the findings here indicate that genomic regulation may play an important, dynamic role in establishing the setpoint of a wide range of neuronal properties and age-dependent changes that are in part regulated by L-VSCCs (1–30). This possibility fits well with growing evidence that expression rates of a number of genes in the central nervous system respond rapidly to multiple normal and pathologic stimuli (57–60).
The present results also appear to have broad implications for the analysis of gene expression–neuronal function correlations in general. This study indicates that quantitative measures of gene expression are feasible in largely intact single neurons that have been functionally characterized. The enhanced accuracy of measures from such neurons should substantially facilitate our ability to identify links between specific genes and defined functional processes. Further, as use of DNA microarray technology becomes more widespread, and perhaps extends to the level of single neurons (61), testing quantitative expression–function correlations could be used to pinpoint which gene, among the hundreds that may be activated, is most closely linked to a specific function. Thus, by virtue of its unique capacity to yield largely intact neurons from animals of any age, the “zipper slice” could well become a preparation of choice for many studies of molecular expression in single, defined neurons.
Acknowledgments
We thank Kelley Secrest and Judith Hower for excellent assistance in the preparation of this manuscript. This research was supported in part by National Institutes of Health Grants AG10836 and AG18228.
Abbreviations
- L-VSCC
L-type voltage-sensitive calcium channel
- RT
reverse transcription
- aCSF
artificial cerebrospinal fluid
- CaM
calmodulin A
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.070056097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.070056097
References
- 1.Tsien R W, Lipscombe D, Madison D, Bley K, Fox A. Trends Neurosci. 1995;18:52–54. [PubMed] [Google Scholar]
- 2.Elliot E M, Malouf A T, Catterall W A. J Neurosci. 1995;15:6433–6444. doi: 10.1523/JNEUROSCI.15-10-06433.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kater S B, Mattson M P, Cohan C, Connor J. Trends Neurosci. 1988;11:315–321. doi: 10.1016/0166-2236(88)90094-x. [DOI] [PubMed] [Google Scholar]
- 4.Koike T, Martin D P, Johnson E M., Jr Proc Natl Acad Sci USA. 1989;86:6421–6425. doi: 10.1073/pnas.86.16.6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipton S A, Kater S B. Trends Neurosci. 1989;12:265–270. doi: 10.1016/0166-2236(89)90026-x. [DOI] [PubMed] [Google Scholar]
- 6.Larmet Y, Dolphin A C, Davies A M. Neuron. 1992;9:563–574. doi: 10.1016/0896-6273(92)90193-h. [DOI] [PubMed] [Google Scholar]
- 7.Gallin W J, Greenberg M E. Curr Opin Neurobiol. 1995;5:367–374. doi: 10.1016/0959-4388(95)80050-6. [DOI] [PubMed] [Google Scholar]
- 8.Bito H, Deisseroth K, Tsien R W. Curr Opin Neurobiol. 1997;7:419–429. doi: 10.1016/s0959-4388(97)80072-4. [DOI] [PubMed] [Google Scholar]
- 9.Moyer J R, Thompson L T, Black J P, Disterhoft J F. J Neurophysiol. 1992;68:2100–2109. doi: 10.1152/jn.1992.68.6.2100. [DOI] [PubMed] [Google Scholar]
- 10.Magee J C, Avery R B, Christie B R, Johnston D. J Neurophysiol. 1996;76:3460–3470. doi: 10.1152/jn.1996.76.5.3460. [DOI] [PubMed] [Google Scholar]
- 11.Christie B R, Eliot L S, Ito K I, Miyakawa H, Johnston D. J Neurophysiol. 1995;73:2553–2557. doi: 10.1152/jn.1995.73.6.2553. [DOI] [PubMed] [Google Scholar]
- 12.Deyo R A, Straub K T, Disterhoft J F. Science. 1989;243:809–811. doi: 10.1126/science.2916127. [DOI] [PubMed] [Google Scholar]
- 13.Disterhoft J F, Moyer J R, Jr, Thompson L T. In: Calcium Hypothesis of Aging and Dementia. Disterhoft J F, Gispen W H, Traber J, Kachaturian Z S, editors. Vol. 747. New York: New York Academy of Sciences; 1994. pp. 382–406. [DOI] [PubMed] [Google Scholar]
- 14.Kapur A, Yeckel M F, Gray R, Johnston D. J Neurophysiol. 1998;79:2181–2190. doi: 10.1152/jn.1998.79.4.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shankar S, Teyler T J, Robbins N. J Neurophysiol. 1998;79:334–341. doi: 10.1152/jn.1998.79.1.334. [DOI] [PubMed] [Google Scholar]
- 16.Norris C M, Halpain S, Foster T C. J Neurophysiol. 1998;80:1567–1570. doi: 10.1152/jn.1998.80.3.1567. [DOI] [PubMed] [Google Scholar]
- 17.Weiss J H, Hartley D M, Koh J, Choi D W. Science. 1990;247:1474–1477. doi: 10.1126/science.247.4949.1474. [DOI] [PubMed] [Google Scholar]
- 18.Catterall W, Epstein P N. Diabetologia. 1992;35:S23–S33. doi: 10.1007/BF00586276. [DOI] [PubMed] [Google Scholar]
- 19.Junti-Berggren L, Larsson O, Rorsman P, Ammala C, Bokvist K, Wahlander K, Nicotera P, Dybukt J, Orrenius S, Hallberg A, et al. Science. 1993;261:86–90. doi: 10.1126/science.7686306. [DOI] [PubMed] [Google Scholar]
- 20.Beenen O H, Mathy M J, Pfaffendorf M, van Zwieten P A. J Hypertens. 1996;14:847–853. doi: 10.1097/00004872-199607000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Calcutt N A, Chaplan S R. Br J Pharmacol. 1997;122:1478–1482. doi: 10.1038/sj.bjp.0701538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith R G, Hamilton S, Hofmann F, Schneider T, Nastaincyk W, Birnbaumer L, Stefani E, Appel S H. N Engl J Med. 1992;327:1721–1728. doi: 10.1056/NEJM199212103272405. [DOI] [PubMed] [Google Scholar]
- 23.Engelhardt J I, Siklos L, Appel S H. J Neuropathol Exp Neurol. 1997;56:21–39. doi: 10.1097/00005072-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Offen D, Halevi S, Orion D, Mosberg R, Stern-Goldberg H, Melamed E, Atlas D. Neurology. 1998;51:1100–1103. doi: 10.1212/wnl.51.4.1100. [DOI] [PubMed] [Google Scholar]
- 25.Landfield P W, Pitler T A. Science. 1984;226:1089–1092. doi: 10.1126/science.6494926. [DOI] [PubMed] [Google Scholar]
- 26.Kerr D S, Campbell L W, Hao S-Y, Landfield P W. Science. 1989;245:1505–1509. doi: 10.1126/science.2781293. [DOI] [PubMed] [Google Scholar]
- 27.Campbell L W, Hao S-Y, Thibault O, Blalock E M, Landfield P W. J Neurosci. 1996;16:6286–6295. doi: 10.1523/JNEUROSCI.16-19-06286.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thibault O, Porter N M, Chen K-C, Blalock E M, Kaminker P G, Clodfelter G V, Brewer L D, Landfield P W. Cell Calcium. 1998;24:417–433. doi: 10.1016/s0143-4160(98)90064-1. [DOI] [PubMed] [Google Scholar]
- 29.Porter N M, Thibault O, Thibault V, Chen K-C, Landfield P W. J Neurosci. 1997;17:5629–5639. doi: 10.1523/JNEUROSCI.17-14-05629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blalock E M, Porter N M, Landfield P W. J Neurosci. 1999;19:8674–8684. doi: 10.1523/JNEUROSCI.19-19-08674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thibault O, Landfield P W. Science. 1996;272:1007–1020. doi: 10.1126/science.272.5264.1017. [DOI] [PubMed] [Google Scholar]
- 32.Hosey M M, Chang F C, O'Callahan C M, Ptasienski J. Ann N Y Acad Sci. 1989;560:27–38. doi: 10.1111/j.1749-6632.1989.tb24076.x. [DOI] [PubMed] [Google Scholar]
- 33.Snutch T P, Leonard J P, Gilbert M M, Lester H A, Davidson N. Proc Natl Acad Sci USA. 1990;87:3391–3395. doi: 10.1073/pnas.87.9.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Catterall W A, DeJongh K, Rotman E, Hell J, Westenbroek R, Dubel S J, Snutch T P. Ann N Y Acad Sci. 1993;681:342–355. doi: 10.1111/j.1749-6632.1993.tb22913.x. [DOI] [PubMed] [Google Scholar]
- 35.Hui A, Ellinor P T, Krizanova O, Wang J J, Diebold R J, Schwartz A. Neuron. 1991;7:35–44. doi: 10.1016/0896-6273(91)90072-8. [DOI] [PubMed] [Google Scholar]
- 36.Herman J P, Chen K-C, Booze R, Landfield P W. Neurobiol Aging. 1998;19:581–587. doi: 10.1016/s0197-4580(98)00099-2. [DOI] [PubMed] [Google Scholar]
- 37.Lambolez B, Audinat E, Bochet P, Crepel F, Rossier J. Neuron. 1992;9:247–258. doi: 10.1016/0896-6273(92)90164-9. [DOI] [PubMed] [Google Scholar]
- 38.Monyer H, Lambolez B. Curr Opin Neurobiol. 1995;5:382–387. doi: 10.1016/0959-4388(95)80052-2. [DOI] [PubMed] [Google Scholar]
- 39.Eberwine J H, Yeh H, Miyashiro K, Cao Y, Nair S, Finnell R, Zettel M, Coleman P. Proc Natl Acad Sci USA. 1992;89:3010–3014. doi: 10.1073/pnas.89.7.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sudweeks S N, Twyman R E. Neurochem Int. 1996;28:137–139. doi: 10.1016/0197-0186(95)00076-3. [DOI] [PubMed] [Google Scholar]
- 41.Bargas J, Howe A, Eberwine J, Cao Y, Surmeier D J. J Neurosci. 1994;14:6667–6686. doi: 10.1523/JNEUROSCI.14-11-06667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sucher N J, Deitcher D L. Neuron. 1995;14:1095–1100. doi: 10.1016/0896-6273(95)90257-0. [DOI] [PubMed] [Google Scholar]
- 43.Baro D J, Levini R M, Kim M T, Willms A R, Lanning C C, Rodriguez H E, Harris-Warrick R M. J Neurosci. 1997;17:6597–6610. doi: 10.1523/JNEUROSCI.17-17-06597.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kay A B, Wong R S K. J Neurosci Methods. 1986;16:227–238. doi: 10.1016/0165-0270(86)90040-3. [DOI] [PubMed] [Google Scholar]
- 45.Steward O, Wallace C S, Lyford G L, Worley P F. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- 46.Baranauskas G, Tkatch T, Surmeier D J. J Neurosci. 1999;19:6394–6404. doi: 10.1523/JNEUROSCI.19-15-06394.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gray R, Fisher R, Spruston N, Johnston D. In: Preparations of the Vertebrate Central Nervous System In Vitro. Jahnsen H, editor. New York: Wiley; 1990. pp. 3–23. [Google Scholar]
- 48.Hamill O P, Marty E, Neher E, Sakmann E, Sigworth F J. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 49.Fisher R E, Gray R, Johnston D. J Neurophysiol. 1990;64:91–104. doi: 10.1152/jn.1990.64.1.91. [DOI] [PubMed] [Google Scholar]
- 50.Hess P, Lansman J B, Tsien R W. Nature (London) 1984;311:538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- 51.Nowycky M C, Fox A P, Tsien R W. Nature (London) 1985;316:440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- 52.Salomon R N, Underwood R, Doyle M V, Wang A, Libby P. Proc Natl Acad Sci USA. 1992;89:2814–2818. doi: 10.1073/pnas.89.7.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bruning J L, Kintz B L. Computational Handbook of Statistics. 3rd Ed. Glenview, IL: HaperCollins; 1987. pp. 180–183. [Google Scholar]
- 54.Sakmann B, Neher E. In: Single Channel Recordings. Sakmann B, Neher E, editors. New York: Plenum; 1983. pp. 37–51. [Google Scholar]
- 55.Blalock E M, Chen K C, Landfield P W, Slevin J T. Soc Neurosci Abs. 1999;25:1111. [Google Scholar]
- 56.Hell J W, Westenbroek R E, Warner C, Ahlijanian M K, Prystay W, Gilbert M M, Snutch T P, Catterall W A. J Cell Biol. 1993;123:949–962. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gall C M. Trends Pharmacol Sci. 1992;13:401–403. doi: 10.1016/0165-6147(92)90123-n. [DOI] [PubMed] [Google Scholar]
- 58.Schreiber S S, Baudry M. Trends Neurosci. 1995;18:446–451. doi: 10.1016/0166-2236(95)94495-q. [DOI] [PubMed] [Google Scholar]
- 59.Kelly M S, Steward O. Rev Neurosci. 1997;8:147–177. doi: 10.1515/revneuro.1997.8.3-4.147. [DOI] [PubMed] [Google Scholar]
- 60.McEwen B S, Alves S E. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 61.Editorial. Nat Neurosci. 1999;2:1. [Google Scholar]