Abstract
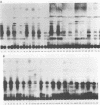
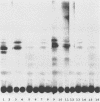
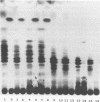
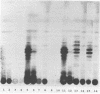
Lipooligosaccharides (Nod metabolites) have been shown to be essential for the successful nodulation of legumes. In strains of Rhizobium leguminosarum bv. trifolii, Nod metabolites were detected predominantly within the cell and to a lesser extent in the periplasmic space and the growth medium. The production, and in particular the excretion, of Nod metabolites was restricted by a range of environmental conditions which are associated with poor nodulation in the field. Lowering the medium pH from 7.0 to 5.0, reducing the phosphate concentration from 1 mM to 5 μM KH2PO4, and lowering the incubation temperature from 28 to 18°C affected the number and relative concentrations of the Nod metabolites made. The form and concentration of the nitrogen source affected the relative concentrations of the Nod metabolites produced and excreted. KNO3 concentrations of >10 mM did not affect cell growth rate but substantially reduced the number of Nod metabolites released. Environmental stresses differentially altered Nod metabolite production and excretion in the same strain carrying different introduced nod regions. Strain ANU845(pWLH1) produced and excreted comparatively fewer Nod metabolites at pH 5.0 and at 18°C than strain ANU845(pRI4003). The excretion but not the production of Nod metabolites by strain ANU845(pRtO32) was dependent on the presence of both nodI and nodJ. Tn5-induced nodI and nodJ mutants did not accumulate any metabolites either outside the cell or within the outer membrane or periplasmic space. Recognition that Nod metabolite accumulation is a complex system of production and excretion, with each component responding differently to changes in environmental conditions, has many consequences, both at the molecular level and in the field. The ability of different strains to produce and release Nod metabolites is likely to be a major determinant of nodule occupancy and should be considered when screening strains suitable for adverse environments.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almendras A. S., Bottomley P. J. Influence of Lime and Phosphate on Nodulation of Soil-Grown Trifolium subterraneum L. by Indigenous Rhizobium trifolii. Appl Environ Microbiol. 1987 Sep;53(9):2090–2097. doi: 10.1128/aem.53.9.2090-2097.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic M. A., Redmond J. W., Batley M., Rolfe B. G. Clovers secrete specific phenolic compounds which either stimulate or repress nod gene expression in Rhizobium trifolii. EMBO J. 1987 May;6(5):1173–1179. doi: 10.1002/j.1460-2075.1987.tb02351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans I. J., Downie J. A. The nodI gene product of Rhizobium leguminosarum is closely related to ATP-binding bacterial transport proteins; nucleotide sequence analysis of the nodI and nodJ genes. Gene. 1986;43(1-2):95–101. doi: 10.1016/0378-1119(86)90012-0. [DOI] [PubMed] [Google Scholar]
- Hollingsworth R. I., Abe M., Sherwood J. E., Dazzo F. B. Bacteriophage-induced acidic heteropolysaccharide lyases that convert the acidic heteropolysaccharides of Rhizobium trifolii into oligosaccharide units. J Bacteriol. 1984 Nov;160(2):510–516. doi: 10.1128/jb.160.2.510-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innes R. W., Hirose M. A., Kuempel P. L. Induction of nitrogen-fixing nodules on clover requires only 32 kilobase pairs of DNA from the Rhizobium trifolii symbiosis plasmid. J Bacteriol. 1988 Sep;170(9):3793–3802. doi: 10.1128/jb.170.9.3793-3802.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K., Bottomley P. J. Influence of Phosphate on the Growth and Nodulation Characteristics of Rhizobium trifolii. Appl Environ Microbiol. 1987 Sep;53(9):2098–2105. doi: 10.1128/aem.53.9.2098-2105.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis-Henderson W. R., Djordjevic M. A. A cultivar-specific interaction between Rhizobium leguminosarum bv. trifolii and subterranean clover is controlled by nodM, other bacterial cultivar specificity genes, and a single recessive host gene. J Bacteriol. 1991 May;173(9):2791–2799. doi: 10.1128/jb.173.9.2791-2799.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis-Henderson W. R., Djordjevic M. A. nodT, a positively-acting cultivar specificity determinant controlling nodulation of Trifolium subterraneum by Rhizobium leguminosarum biovar trifolii. Plant Mol Biol. 1991 Apr;16(4):515–526. doi: 10.1007/BF00023418. [DOI] [PubMed] [Google Scholar]
- McIver J., Djordjevic M. A., Weinman J. J., Bender G. L., Rolfe B. G. Extension of host range of Rhizobium leguminosarum bv. trifolii caused by point mutations in nodD that result in alterations in regulatory function and recognition of inducer molecules. Mol Plant Microbe Interact. 1989 May-Jun;2(3):97–106. doi: 10.1094/mpmi-2-097. [DOI] [PubMed] [Google Scholar]
- Mullen M. D., Israel D. W., Wollum A. G. Effects of Bradyrhizobium japonicum and Soybean (Glycine max (L.) Merr.) Phosphorus Nutrition on Nodulation and Dinitrogen Fixation. Appl Environ Microbiol. 1988 Oct;54(10):2387–2392. doi: 10.1128/aem.54.10.2387-2392.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nap J. P., Bisseling T. Developmental biology of a plant-prokaryote symbiosis: the legume root nodule. Science. 1990 Nov 16;250(4983):948–954. doi: 10.1126/science.250.4983.948. [DOI] [PubMed] [Google Scholar]
- Richardson A. E., Simpson R. J., Djordjevic M. A., Rolfe B. G. Expression of Nodulation Genes in Rhizobium leguminosarum biovar trifolii Is Affected by Low pH and by Ca and Al Ions. Appl Environ Microbiol. 1988 Oct;54(10):2541–2548. doi: 10.1128/aem.54.10.2541-2548.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres B., Van De Wiel C., Zalensky A., Horvath B., Spaink H., Van Eck H., Zwartkruis F., Wolters A. M., Gloudemans T., Van Kammen A. The ENOD12 gene product is involved in the infection process during the pea-Rhizobium interaction. Cell. 1990 Jan 26;60(2):281–294. doi: 10.1016/0092-8674(90)90743-x. [DOI] [PubMed] [Google Scholar]
- Singleton P. W., Bohlool B. B. Effect of salinity on nodule formation by soybean. Plant Physiol. 1984 Jan;74(1):72–76. doi: 10.1104/pp.74.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaink H. P., Aarts A., Stacey G., Bloemberg G. V., Lugtenberg B. J., Kennedy E. P. Detection and separation of Rhizobium and Bradyrhizobium Nod metabolites using thin-layer chromatography. Mol Plant Microbe Interact. 1992 Jan-Feb;5(1):72–80. doi: 10.1094/mpmi-5-072. [DOI] [PubMed] [Google Scholar]
- Spaink H. P. Rhizobial lipo-oligosaccharides: answers and questions. Plant Mol Biol. 1992 Dec;20(5):977–986. doi: 10.1007/BF00027167. [DOI] [PubMed] [Google Scholar]
- Spaink H. P., Sheeley D. M., van Brussel A. A., Glushka J., York W. S., Tak T., Geiger O., Kennedy E. P., Reinhold V. N., Lugtenberg B. J. A novel highly unsaturated fatty acid moiety of lipo-oligosaccharide signals determines host specificity of Rhizobium. Nature. 1991 Nov 14;354(6349):125–130. doi: 10.1038/354125a0. [DOI] [PubMed] [Google Scholar]
- Truchet G., Debellé F., Vasse J., Terzaghi B., Garnerone A. M., Rosenberg C., Batut J., Maillet F., Dénarié J. Identification of a Rhizobium meliloti pSym2011 region controlling the host specificity of root hair curling and nodulation. J Bacteriol. 1985 Dec;164(3):1200–1210. doi: 10.1128/jb.164.3.1200-1210.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Brussel A. A., Zaat S. A., Cremers H. C., Wijffelman C. A., Pees E., Tak T., Lugtenberg B. J. Role of plant root exudate and Sym plasmid-localized nodulation genes in the synthesis by Rhizobium leguminosarum of Tsr factor, which causes thick and short roots on common vetch. J Bacteriol. 1986 Feb;165(2):517–522. doi: 10.1128/jb.165.2.517-522.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]