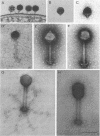
Abstract
Recent reports documenting very high viral abundances in seawater have led to increased interest in the role of viruses in aquatic environments and a resurgence of the hypothesis that viruses are significant agents of bacterial mortality. Synechococcus spp., small unicellular cyanobacteria that are important primary producers at the base of the marine food web, were used to assess this hypothesis. We isolated a diverse group of Synechococcus phages that at times reach titers of between 103 and 104 cyanophages per ml in both inshore and offshore waters. However, despite their diversity and abundance, we present evidence in support of the hypothesis that lytic phages have a negligible effect in regulating the densities of marine Synechococcus populations. Our results indicate that these bacterial communities are dominated by cells resistant to their co-occurring phages and that these viruses are maintained by scavenging on the relatively rare sensitive cells in these communities.
Full text
PDF


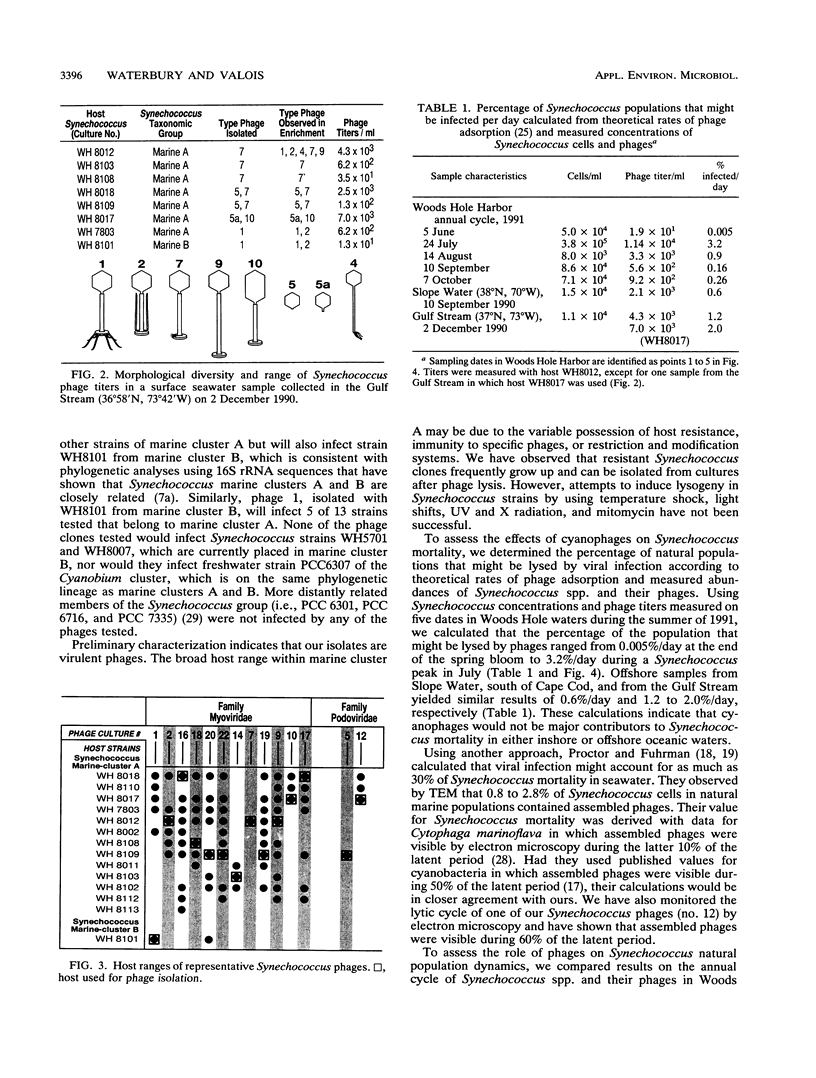
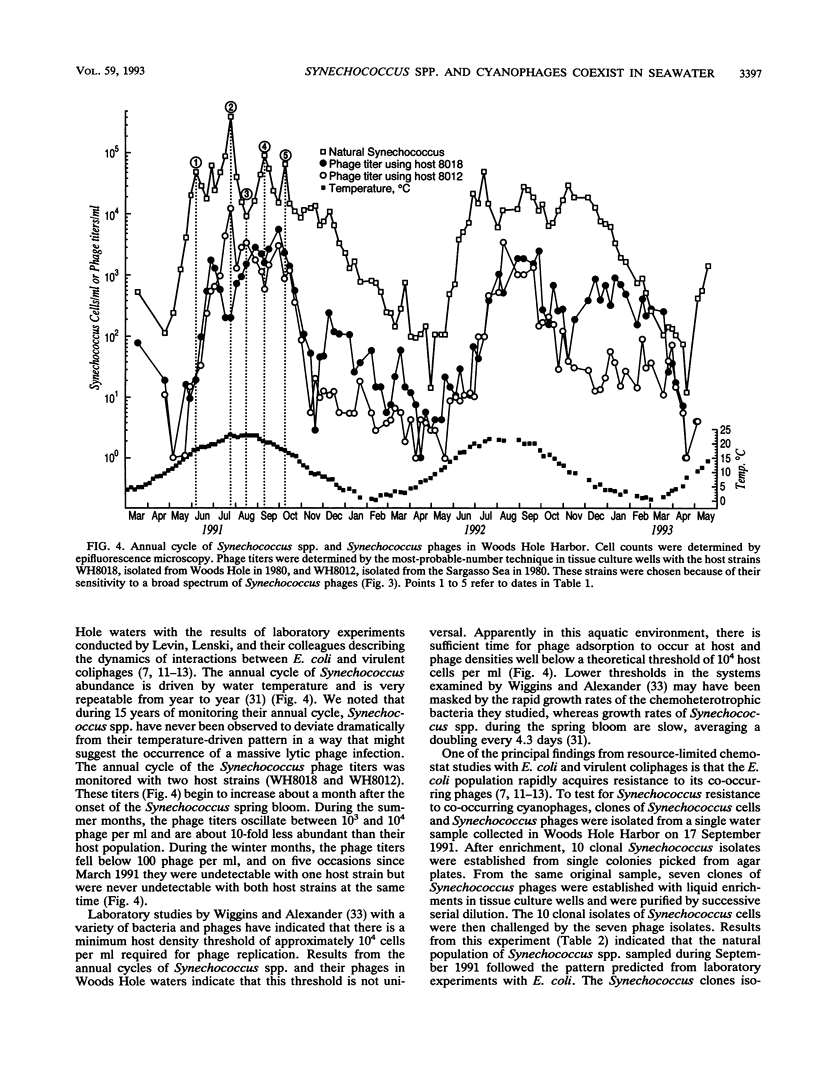
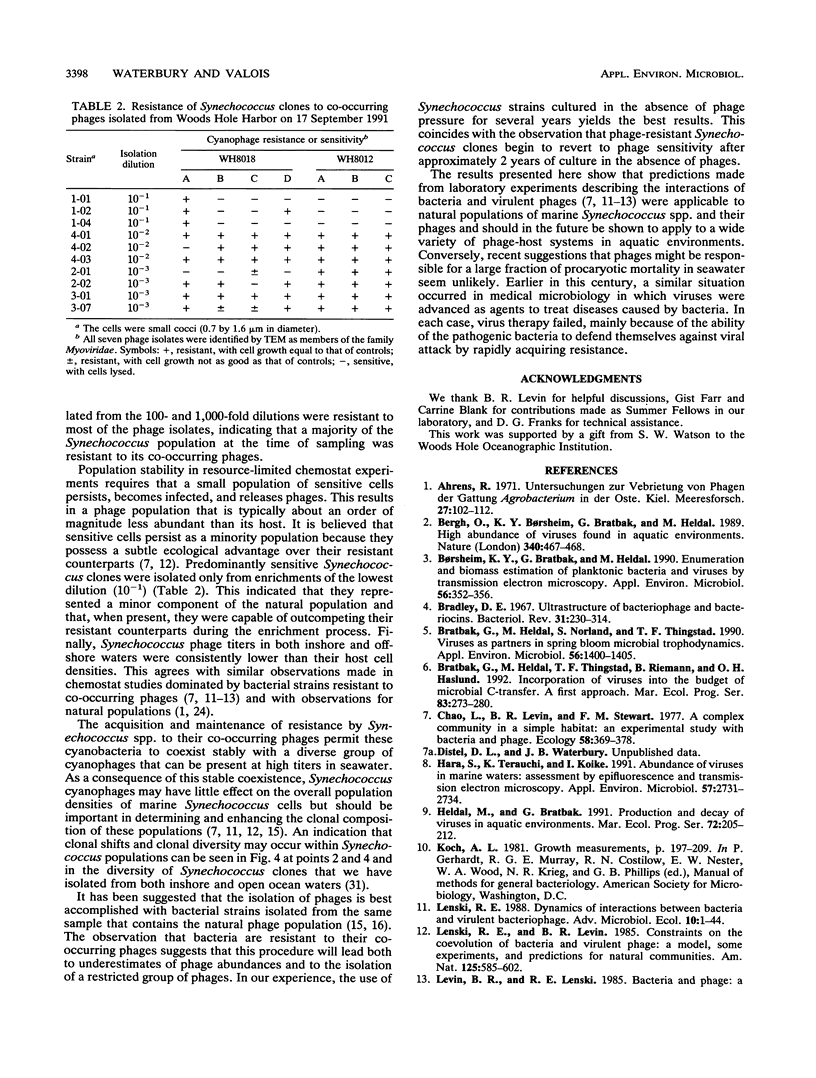

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergh O., Børsheim K. Y., Bratbak G., Heldal M. High abundance of viruses found in aquatic environments. Nature. 1989 Aug 10;340(6233):467–468. doi: 10.1038/340467a0. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratbak G., Heldal M., Norland S., Thingstad T. F. Viruses as partners in spring bloom microbial trophodynamics. Appl Environ Microbiol. 1990 May;56(5):1400–1405. doi: 10.1128/aem.56.5.1400-1405.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Børsheim K. Y., Bratbak G., Heldal M. Enumeration and biomass estimation of planktonic bacteria and viruses by transmission electron microscopy. Appl Environ Microbiol. 1990 Feb;56(2):352–356. doi: 10.1128/aem.56.2.352-356.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara S., Terauchi K., Koike I. Abundance of viruses in marine waters: assessment by epifluorescence and transmission electron microscopy. Appl Environ Microbiol. 1991 Sep;57(9):2731–2734. doi: 10.1128/aem.57.9.2731-2734.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews R. E. Third report of the International Committee on Taxonomy of Viruses. Classification and nomenclature of viruses. Intervirology. 1979;12(3-5):129–296. doi: 10.1159/000149081. [DOI] [PubMed] [Google Scholar]
- Padan E., Shilo M. Cyanophages-viruses attacking blue-green algae. Bacteriol Rev. 1973 Sep;37(3):343–370. doi: 10.1128/br.37.3.343-370.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safferman R. S., Cannon R. E., Desjardins P. R., Gromov B. V., Haselkorn R., Sherman L. A., Shilo M. Classification and nomenclature of viruses of cyanobacteria. Intervirology. 1983;19(2):61–66. doi: 10.1159/000149339. [DOI] [PubMed] [Google Scholar]
- Suttle C. A., Chan A. M., Cottrell M. T. Use of ultrafiltration to isolate viruses from seawater which are pathogens of marine phytoplankton. Appl Environ Microbiol. 1991 Mar;57(3):721–726. doi: 10.1128/aem.57.3.721-726.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine A. F., Chapman G. B. Fine structure and host-virus relationship of a marine bacterium and its bacteriophage. J Bacteriol. 1966 Nov;92(5):1535–1554. doi: 10.1128/jb.92.5.1535-1554.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterbury J. B., Stanier R. Y. Patterns of growth and development in pleurocapsalean cyanobacteria. Microbiol Rev. 1978 Mar;42(1):2–44. doi: 10.1128/mr.42.1.2-44.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins B. A., Alexander M. Minimum bacterial density for bacteriophage replication: implications for significance of bacteriophages in natural ecosystems. Appl Environ Microbiol. 1985 Jan;49(1):19–23. doi: 10.1128/aem.49.1.19-23.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]