Abstract
Context
Although office-based opioid treatment with buprenorphine (OBOT-B) has been successfully implemented in primary care settings in the US, its use has not been reported in homeless patients.
Objective
To characterize the feasibility of OBOT-B in homeless relative to housed patients.
Design
A retrospective record review examining treatment failure, drug use, utilization of substance abuse treatment services, and intensity of clinical support by a nurse care manager (NCM) among homeless and housed patients in an OBOT-B program between August 2003 and October 2004. Treatment failure was defined as elopement before completing medication induction, discharge after medication induction due to ongoing drug use with concurrent nonadherence with intensified treatment, or discharge due to disruptive behavior.
Results
Of 44 homeless and 41 housed patients enrolled over 12 months, homeless patients were more likely to be older, nonwhite, unemployed, infected with HIV and hepatitis C, and report a psychiatric illness. Homeless patients had fewer social supports and more chronic substance abuse histories with a 3- to 6-fold greater number of years of drug use, number of detoxification attempts and percentage with a history of methadone maintenance treatment. The proportion of subjects with treatment failure for the homeless (21%) and housed (22%) did not differ (P = .94). At 12 months, both groups had similar proportions with illicit opioid use [Odds ratio (OR), 0.9 (95% CI, 0.5–1.7) P = .8], utilization of counseling (homeless, 46%; housed, 49%; P = .95), and participation in mutual-help groups (homeless, 25%; housed, 29%; P = .96). At 12 months, 36% of the homeless group was no longer homeless. During the first month of treatment, homeless patients required more clinical support from the NCM than housed patients.
Conclusions
Despite homeless opioid dependent patients’ social instability, greater comorbidities, and more chronic drug use, office-based opioid treatment with buprenorphine was effectively implemented in this population comparable to outcomes in housed patients with respect to treatment failure, illicit opioid use, and utilization of substance abuse treatment.
Key words: buprenorphine, drug dependence, primary care, homelessness
INTRODUCTION
Opioid abuse persists as a pervasive public health problem in the United States, both heroin1 and prescription opioid analgesics.1,2 Opioid agonist treatment with methadone or buprenorphine is effective for treating opioid dependence.3–12 With the advent of sublingual buprenorphine for the treatment of opioid dependence, primary care physicians in the United States gained the opportunity to effectively treat opioid-dependent patients in primary medical care settings, commonly referred to as office-based opioid treatment (OBOT).13,14
In 2003, the primary care clinic at Boston Medical Center (BMC) implemented an OBOT with buprenorphine (OBOT-B) program employing collaborative care between physicians and a nurse care manager (NCM).15 All patients in the BMC primary care clinic OBOT-B program were required to have stable housing, as clinical guidelines recommend a stable social environment as an entry criterion for OBOT-B.16,17 Using social stability as a criterion for OBOT-B precludes homeless persons, a population with a high prevalence of addiction,18–21 leading to a high risk of illness and death.22–25
Unique challenges confront homeless individuals engaging in substance abuse treatment,26 which likely contribute to their high rates of treatment failure.27,28 Characteristics of homeless persons are correlated with relapse: lack of social support; unstable living environment; and longer duration of drug dependence.29,30 However, research has shown that homeless persons’ success in substance abuse treatment can increase under supportive circumstances.30,31 Furthermore, despite limited literature on methadone treatment in homeless populations, published data suggest greater success with enhanced access to32 and unconventional methods of treatment (e.g., medical care provision in homeless health care settings).33 Although homeless opioid dependent individuals may benefit from buprenorphine treatment, clinical guidelines have excluded them.
This retrospective cohort study characterizes the feasibility of office-based opioid treatment with buprenorphine (OBOT-B) in a homeless population. We describe a customized model of OBOT-B care and outcomes in homeless compared to housed patients.
METHODS
Program Description
Model of Care In August 2003, OBOT-B was implemented in an urban academic medical center for homeless patients in a homeless clinic and for housed patients in a primary care clinic. Both patient groups were treated with collaborative care between qualified primary care physicians (PCPs) and a single nurse care manager (NCM). All patients were referred by their respective clinics and were to receive maintenance buprenorphine treatment for at least 12 months. Homelessness was defined as spending 1 or more weeks on the street or in a shelter within the previous 3 months. Upon referral by the PCP, homeless and housed patients were admitted successively or placed on a wait list when the practice was at capacity due to the federally mandated 30-patient-per-practice limit at the time of the study. Our model of care included 4 stages of treatment: (1) Determination of eligibility; (2) Medication induction; (3) Medication stabilization; (4) Treatment maintenance.
Determination of Eligibility Assessment protocols were similar for homeless and housed groups. OBOT-B, a less structured treatment option compared to methadone maintenance treatment, required a patient assessment for appropriateness. OBOT-B appropriateness was determined by adherence with assessment appointments, mental health stability [i.e., ability to comprehend and consent to OBOT-B protocols, chronic mental illness with ongoing psychiatric care and no acute mental health issues (e.g., suicidal ideation)], absence of painful conditions requiring opioid analgesics, absence of medical contraindications (e.g., pregnancy or liver function tests greater than five times the normal level), and absence of alcohol or other drug co-dependence. Patients with sporadic alcohol or other drug use remained eligible. Patients on methadone maintenance doses greater than 30 mg per day were excluded based on current practice guidelines.16 If deemed appropriate for OBOT-B, patients received a comprehensive assessment by the NCM that included a detailed medical, psychiatric, and substance abuse history including documentation of DSM-IV diagnosis of opioid dependence. Social history included employment and homelessness status. Admission lab work included a complete blood count, electrolytes, renal and liver function tests, hepatitis A, B, and C serologies, and urine toxicology for opioids (including oxycodone for patients with a history of oxycodone abuse), cocaine, benzodiazepines, barbiturates, and amphetamines. All patients were tested for tuberculosis exposure and were offered HIV testing and counseling. Treatment consents and individualized treatment plans were reviewed and signed by both patient and NCM. At a separate appointment, PCPs reviewed the NCM assessment and treatment plan, performed a complete physical examination, and wrote the initial buprenorphine prescription. Patients were strongly encouraged to utilize other substance abuse services, including addiction counseling and mutual-help groups [e.g., Narcotics Anonymous (NA)].
Medication Induction Induction, occurring during the first 3 days of treatment, differed for homeless and housed patients. Homeless patients received “office induction,” with up to 8 hours observation on day 1 by the NCM for the following: signs of opioid withdrawal, dose administration, and response to medication. Homeless patients left the office with a nighttime dosing protocol to be used if withdrawal recurred and then returned daily for the next 2 days for dose adjustments. In contrast, because all housed patients were reachable by telephone, they received “home induction” by following a 3-day dosing protocol at home. The 3-day protocol gave specific dosing schedules with a maximum daily dose for each day (i.e., max dose, 8, 16, and 24 mg for days 1, 2, and 3, respectively). The NCM called all housed patients at home before the first buprenorphine dose was taken and then at least daily during the 3-day induction. The NCM was available during business hours for drop-in visits and 24 hours a day via cell phone.
Medication Stabilization Stabilization, occurring during days 4 through 14 of treatment, differed for homeless and housed patients. Homeless patients presented to the clinic daily, except weekends, for observed dosing and assessment while housed patients were seen twice per week but had at least daily NCM phone contact.
Treatment Maintenance Ongoing monitoring for drug use and treatment adherence, occurring beyond day 14 of treatment, was based on individual patient needs in both groups. Buprenorphine prescriptions were provided in 1 to 4 week amounts determined by the patient’s ability to safely secure medications. For example, 1-week amounts for patients without a secure storage place and 2–4 week amounts for patients with a secure storage place. Patients were seen at least monthly by the NCM and at least every 6 months by the PCP. Patients were expected to respond to unscheduled “call-backs” to the clinic for urine toxicology, observed dosing, pill counts, and revisions of treatment plans as needed. The need for “call-backs” was based on the NCM or PCP’s clinical suspicion that the patient may have relapsed or may be diverting their medication based on abnormal urine toxicology, reports of lost pills, requests for early refills, or missed appointments. Urine was tested for opioids (including oxycodone for patients with a history of oxycodone abuse) and other drugs (including cocaine, benzodiazepines, barbiturates, and amphetamines) as well as buprenorphine at least every 3 months. Intensified treatment (i.e., substance abuse counseling) was required for patients with ongoing use of opioids, other drugs, or alcohol.
Patient Characteristics
Upon entry into OBOT-B, patients’ demographics, employment, homelessness status, involvement of socially supportive individuals, opioid, other drug and alcohol use, and substance abuse, medical and psychiatric histories were recorded.
Outcome Assessments
Time to Treatment Failure Our primary outcome was time to “treatment failure” defined as any of the following: elopement before completing medication induction (elopement did not occur after induction); discharge after medication induction due to ongoing use of opioids, other drugs or alcohol with concurrent nonadherence with intensified substance abuse treatment; or discharge due to disruptive behavior (e.g., threatening staff, theft of clinic property).Secondary outcomes included reasons for leaving the OBOT-B program (including treatment failure and successful program departures), illicit opioid and other drug use, number of NCM contacts, utilization of recommended care and social indicators (e.g., homelessness and employment status).
Reasons for Leaving the OBOT-B Program All patients who left the program before 12 months were categorized into one of the following 2 groups: “treatment failure” (defined above); or “successful program departure.” Patients classified as “successful program departure” left the program because they relocated and were transferred to another OBOT program, they needed a more structured treatment setting and were transferred to a methadone maintenance program, or they were fully adherent with treatment for at least 4 months and were approved for a medication taper by both the NCM and PCP.
Illicit Drug Use Both planned and “call-back” urine toxicology were conducted at least once every 3 months. In each assessment window (i.e., at entry, 3, 6, 9, and 12 months of treatment), the test that was closest, yet prior, to the timepoint was reported, in an attempt to obtain a measure of abstinence that was not biased by the number of urine toxicology tests performed. Urine toxicology tests were mostly unsupervised but measures were taken to try to minimize falsified tests (e.g., testing for buprenorphine metabolites, urine temperature, and creatinine concentration). Urine collections were supervised when the NCM suspected falsified tests due to the following circumstances: patient had a recent history of abnormal urine tests; specimens were cold or diluted; patient demonstrated aberrant behavior (e.g., missed clinic appointments, requested early medication refill). The following 3 outcomes were determined by record review: number of NCM contacts (phone calls and clinic visits during each month of treatment); utilization of recommended care (involvement in substance abuse counseling and/or mutual-help groups defined by at least weekly attendance for 1 or more months before record review); and social indicators [homelessness, employment status (self-reported employment for at least 1 month) and presence of social supports (family member or friend actively involved in the patient’s substance abuse rehabilitative progress and in contact with the NCM within the previous 3 months)].For all patients who left the OBOT-B program (treatment failure and successful program departure), follow-up data post-departure were not collected.
Analysis
Descriptive statistics (e.g., means and proportions) were used to characterize homeless and housed groups. Exploratory, hypothesis-generating tests were then performed to compare process and outcome measures between groups. Chi-square or Fisher’s exact tests were used to compare dichotomous outcomes and t-tests or Wilcoxon rank sum tests were used to compare continuous outcomes between groups. NCM contacts were described for each group using the mean number of contacts per patient for each month of follow-up. Poisson regression models were constructed to estimate the rate ratio of NCM contacts in the homeless relative to housed groups. Generalized estimating equations (GEE) regression models were used to examine the association between homelessness status and drug use. Data collected from entry, 3, 6, 9, and 12-month timepoints were analyzed. The proportions of treatment failures were estimated using the Kaplan–Meier method and compared between groups using the log-rank test. Successful program departures were censored at their date of departure, as follow-up data were not available beyond that date. Reported P-values are two-tailed, and a P-value of less than .05 was considered statistically significant. All analyses were run using SAS statistical software.34 This research was approved by the Institutional Review Board at Boston University Medical Center.
RESULTS
Patient Characteristics
During the 12 months examined, 44 homeless and 41 housed patients were enrolled. The homeless group had fewer males, fewer whites, were older, and less employed compared to the housed group. There was a dramatic difference in social supports, essentially nonexistent for the homeless patients but present for almost all housed patients. Similarly, comorbidities were clearly different in the homeless versus the housed group with 95% reporting psychiatric illness versus 54%, 30% HIV-infected versus 5%, and 95% hepatitis C infected versus 44%, respectively. At time of study entry, 84% of the homeless patients were dependent on heroin with none dependent on oxycodone versus 63% heroin dependent and 27% oxycodone dependent in the housed patients. The remaining 16% of the homeless and 10% of housed patients were transferred from methadone maintenance treatment. Homeless patients also had more chronic substance abuse histories with a 3- to 6-fold greater number of years of drug use, number of detoxification attempts, and percentage with a history of methadone maintenance treatment (Table 1).
Table 1.
Comparison of Homeless and Housed Patients at Time of Entry into Office-Based Opioid Treatment with Buprenorphine
| Homeless (N = 44) | Housed (N = 41) | P-value | |
|---|---|---|---|
| Demographics | |||
| Male (%) | 59 | 76 | .11 |
| Race/ethnicity | <.001 | ||
| White (%) | 41 | 85 | |
| Hispanic/Latino (%) | 34 | 2 | |
| Black/African American (%) | 25 | 12 | |
| Mean age years (SD) | 42 (9.1) | 34 (10.4) | <.001 |
| Employed (%) | 5 | 34 | <.001 |
| Involvement of social support in care (%) | 2 | 90 | <.001 |
| Comorbidity | |||
| Self-reported psychiatric illness (%) | 95 | 54 | <.001 |
| HIV-infected (%) | 30 | 5 | .003 |
| Hepatitis C-infected (%) | 95 | 44 | <.001 |
| Substance abuse history | |||
| Opioid at admission | .001 | ||
| Heroin (%) | 84 | 63 | |
| Sustained-release oxycodone (%) | 0 | 27 | |
| Methadone maintenance (%) | 16 | 10 | |
| Any methadone maintenance history (%) | 59 | 10 | <.001 |
| Median years drug use (range) | 15 (5–30) | 5 (2–12) | <.001 |
| Median detoxification attempts (range) | 18 (5–40) | 5 (0–20) | <.001 |
Patient Outcomes
The estimated proportion of subjects with treatment failure was 21% for the homeless and 22% for the housed. Homeless and housed patients did not differ in their risk for treatment failure during follow-up (P = .94) (Fig. 1).
Figure 1.
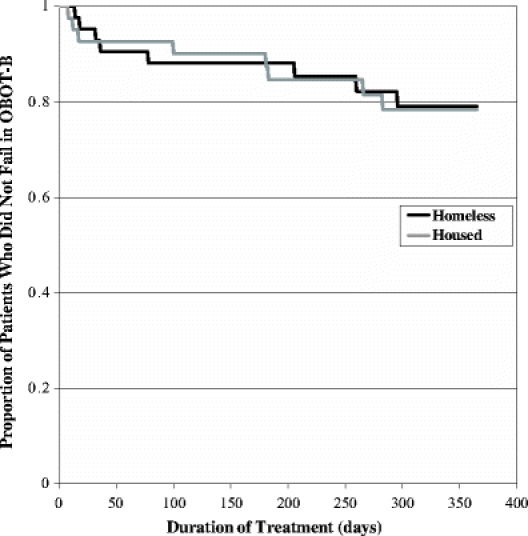
Kaplan–Meier estimates of the proportion of homeless and housed patients who did not fail office-based opioid treatment with buprenorphine. P = .94 for the comparison between homeless and housed subjects by the log-rank test.
Mean duration of OBOT-B retention was 9 months for both homeless and housed groups. The percentage of patients remaining in OBOT-B (i.e., had not failed, transferred or tapered) at the 3-, 6-, 9- and 12-month timepoints was 77%, 73%, 70%, and 55% in the homeless group and 93%, 80%, 68%, and 61% in the housed group. The number of patients who left treatment and the reasons for leaving treatment before 12-months, including the treatment failure and successful program departure groups, appeared similar for both groups (Table 2).
Table 2.
Reasons for Leaving Office-Based Opioid Treatment with Buprenorphine Among Homeless (n = 20*) and Housed (n = 17*) Patients
| Homeless N (%) | Housed N (%) | |
|---|---|---|
| Treatment failure | ||
| Elopement during induction†† | 3 (15) | 3 (18) |
| Ongoing drug use and treatment nonadherence§ | 3 (15) | 5 (29) |
| Disruptive behavior// | 2 (10) | 0 (0) |
| Successful program departures | ||
| Transfer to another OBOT-B program | 1 (5) | 0 (0) |
| Transfer to methadone maintenance program | 5 (25) | 4 (24) |
| Successful taper¶ | 6 (30) | 5 (29) |
*Of the 44 homeless and 41 housed subjects who entered the study, 20 homeless and 17 housed subjects did not remain in the program for 12 months.
††No elopement occurred after induction period.
§Ongoing use of opioids or other drugs with concurrent nonadherence of intensified treatment.
//Including threatening staff and theft of clinic property.
¶Treatment adherence including no drug use for at least 4 months, followed by successful tapering off of the program.
Of the patients remaining in treatment at 3 months, urine samples were positive for illicit opioids in 11% of homeless and 20% of housed patients and at 12 months positive in only 4% in both groups. At entry, urines were positive for “other drugs” in 34% of homeless and 32% of housed patients and of those remaining in treatment at 12 months, positive in only 8% in both groups. Over the 12-month period, no significant associations were found between homelessness status and use of opioids [Odds ratio (OR) 0.9 (95% CI, 0.5–1.7); P = .8] or other drugs [OR 1.7 (95% CI, 0.7–4.3); P = .2).
The mean number of NCM contacts per patient was estimated by homelessness status for each month of follow-up (Fig. 2). During the first month of treatment (induction and stabilization), the rate of NCM contacts per patient in the homeless group was 1.7 times that in the housed group (95% CI = 1.48–1.95; P < .0001). At 12 months, 46% of homeless and 49% of housed patients reported at least weekly receipt of substance abuse counseling and 25% of homeless and 29% of housed patients reported at least weekly mutual-help group attendance (Table 3). At 12 months, of the 44 homeless patients originally enrolled, 16 (36%) no longer met the criteria for homelessness. Employment increased for both homeless and housed patients that remained in the program for 12 months.
Figure 2.

Mean number of monthly nurse care manager (NCM) contacts per homeless and housed patient over 12 months of office-based opioid treatment with buprenorphine. *RR = 1.7 (95% CI = 1.48–1.95); P < .0001 (homeless vs housed in month #1).
Table 3.
Outcomes of Homeless (N = 44) and Housed (N = 41) Patients after 12-months of Office-Based Opioid Treatment with Buprenorphine
| Homeless N (%) | Housed N (%) | P-value | |
|---|---|---|---|
| Attending counseling† | 0.95 | ||
| Yes | 20 (46) | 20 (49) | |
| No | 4 (9) | 4 (10) | |
| Unknown | 20 (46) | 17 (42) | |
| Attending mutual help groups† | 0.96 | ||
| Yes | 11 (25) | 12 (29) | |
| No | 13 (30) | 12 (29) | |
| Unknown | 20 (46) | 17 (42) | |
| Currently homeless†† | 0.03 | ||
| Yes | 8 (18) | 1 (2) | |
| No | 16 (36) | 23 (56) | |
| Unknown | 20 (46) | 17 (42) | |
| Currently employed§ | 0.07 | ||
| Yes | 17 (39) | 23 (56) | |
| No | 7 (16) | 1 (2) | |
| Unknown | 20 (46) | 17 (42) | |
| Involvement of social support in care// | 0.50 | ||
| Yes | 22 (50) | 24 (59) | |
| No | 2 (5) | 0 (0) | |
| Unknown | 20 (46) | 17 (42) |
†Weekly attendance for 1 or more months.
††Spending 1 or more weeks on the street or in a shelter within the previous 3 months.
§Maintaining employment for 1 or more months.
//Family member or friend actively involved in the patient’s substance abuse rehabilitative progress and in contact with the NCM within the previous 3 months.
DISCUSSION
Office-based opioid treatment with buprenorphine (OBOT-B) was effectively implemented in homeless patients with outcomes comparable to housed patients. When compared to housed patients, homeless patients appeared to have a similar proportion of treatment successes (i.e., retention in treatment, successful program departure, decreased drug use, and utilization of counseling and mutual help groups), despite having more chronic substance abuse histories (e.g., greater number of years of drug use, detoxification attempts, and history of methadone maintenance treatment), more medical and psychiatric comorbidities, and less social support. Employment and housing increased substantially among homeless patients. Homeless patients required more clinical support by the Nurse Care Manager (NCM) than housed patients during the first month of treatment. However, notwithstanding the increased support required, feasibility of OBOT-B care to homeless patients was demonstrated and outcomes appeared comparable to a housed group.
These findings conflict with previous reports of poorer addiction treatment outcomes in homeless populations,27,28,32 but are consistent with studies in which homeless-specific interventions improved addiction outcomes. Milby et al. randomized 176 homeless substance abusers to either usual or enhanced care. Enhanced care entailed daily psychoeducational groups and weekly individual counseling, plus abstinence-contingent employment and housing starting at month 4 of treatment. Subjects receiving enhanced care had significantly lower rates of alcohol and drug use during the 12 months of follow-up.31 Kertesz et al. found an association between post-detoxification stabilization programs and time to substance use post-discharge from a residential detoxification program for homeless persons with addictions.30
This study provides further evidence that addiction treatment in homeless populations can yield effective results. It suggests that more than the usual resources, such as availability of a NCM, may be required for this population when pursuing substance abuse treatment.
The availability of a NCM also allowed us to implement a home-based medication induction protocol, which is not currently recommended by national guidelines, through daily phone contact between the NCM and housed patients.
Several limitations to this study should be considered. While data were collected prospectively during clinical care using a carefully designed medical record, the study was retrospective. In addition, follow-up data were not available once patients departed the program. However, of patients who left the program earlier than 12 months, the reasons for leaving (i.e., treatment failure vs successful program departure) appeared similar between homeless and housed groups. To assess the value of an OBOT-B program in homeless persons, one ideally would compare outcomes with homeless persons requesting substance abuse treatment who did not get access to buprenorphine. However, this study was not intended to retest the efficacy of buprenorphine treatment, but rather, evaluate the feasibility of delivering this known effective treatment in a homeless population. Thus, the comparison group in this study was non-homeless persons receiving buprenorphine. An experienced, skilled NCM played an essential role in caring for patients in the programs described. Generalizability of such a model may depend on skills of such a key individual. Generalizability may also depend on the level of collaboration among practitioners within the microsystem in which the care is delivered.35 The provider collaboration component of the OBOT-B program was not explicitly measured in this study. Finally, although the total number of patients in this study is small, a consequence of the federal law limiting the number of patients receiving buprenorphine in a single clinical practice, the findings are robust and statistically significant.
In conclusion, office-based opioid treatment with buprenorphine (OBOT-B) can be effective in homeless patients. In this study, despite homeless patients’ greater comorbidities, less social support, and more chronic substance use histories, their outcomes in terms of treatment failure and illicit drug use were comparable to housed patients. Social benefits, specifically gaining access to housing and employment, occurred in a surprisingly high percentage of the homeless OBOT-B patients. Beyond individual clinical benefit, potentially unmeasured public health benefits regarding risk behaviors and health care utilization need to be further assessed. Using appropriate models of care, buprenorphine treatment for opioid dependence among homeless persons should be implemented.
Acknowledgements
Preliminary results of this study were presented at the 28th annual meeting of the Society of General Internal Medicine in New Orleans on May 11–14, 2005, the 67th annual national meeting of the College on Problems of Drug Dependence in Orlando, FL on June 18–23, 2005, and at the 29th annual meeting of the Association for Medical Education and Research in Substance Abuse in Bethesda, MD on October 27–29, 2005. Data management was provided by Michael Winter MPH and Jacqueline Ashba MA, MPH, at the Data Coordinating Center, Boston University School of Public Health, Boston, MA. Support for these programs and their evaluation was provided by the Massachusetts Department of Public Health: Bureau of Substance Abuse Services and HIV/AIDS Bureau.
Potential Financial Conflicts of Interest None disclosed.
References
- 1.US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration (SAMHSA). Results from the 2004 National Survey on Drug Use & Health. Available at http://oas.samhsa.gov/NSDUH/2k4NSDUH/2k4results/2k4results.htm#ch2. Accessed July 11, 2006.
- 2.US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration (SAMHSA). The DASIS Report: Non-Heroin Opiate Admissions, 2003. Results from the Drug and Alcohol Services Information System (DASIS). 2003. Available at http://oas.samhsa.gov/2k6/opiatesTX/opiatesTX.cfm. Accessed July 11, 2006.
- 3.National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction. Effective medical treatment of opiate addiction. JAMA. 1998;280:1936–43. [DOI] [PubMed]
- 4.Johnson RE, Jaffe JH, and Fudala PJ. A controlled trial of buprenorphine treatment for opioid dependence. JAMA. 1992;267:2750–55. [DOI] [PubMed]
- 5.Ling W, Wesson DR, Charuvastra C, and Klett CJ. A controlled trial comparing buprenorphine and methadone maintenance in opioid dependence. Arch Gen Psychiatry. 1996;53:401–7. [DOI] [PubMed]
- 6.Ball JC and Ross A. The Effectiveness of Methadone Maintenance Treatment. New York, NY: Springer—Berlin Heidelberg New York; 1991.
- 7.Joseph H, Stancliff S, and Langrod J. Methadone maintenance treatment (MMT): a review of historical and clinical issues. Mt Sinai J Med. 2000;67:347–64. [PubMed]
- 8.O’Connor PG and Fiellin DA. Pharmacologic treatment of heroin-dependent patients. Ann Intern Med. 2000;133:40–54. [DOI] [PubMed]
- 9.Institute of Medicine. Federal regulation of methadone treatment. Washington, DC: National Academy of Medicine; 1995.
- 10.Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, and Bigelow GE. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med. 2000;343:1290–7. [DOI] [PubMed]
- 11.Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349:949–58. [DOI] [PubMed]
- 12.Kakko J, Svanborg KD, Kreek MJ, and Heilig M. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomised, placebo-controlled trial. Lancet. 2003;361:662–8. [DOI] [PubMed]
- 13.Fiellin DA and O’Connor PG. New federal initiatives to enhance the medical treatment of opioid dependence. Ann Intern Med. 2002;137:688–92. [DOI] [PubMed]
- 14.Stein MD, Cioe P, and Friedmann PD. Buprenorphine retention in primary care. J Gen Intern Med. 2005;20:1038–41. [DOI] [PMC free article] [PubMed]
- 15.Alford DP, Saitz R, LaBelle CT, and Samet JH. Buprenorphine initiation and maintenance in primary care: a successful interdisciplinary approach. J Gen Intern Med. 2004;19(suppl):103.
- 16.Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction, Treatment Improvement Protocol (TIP) Series 40. DHHS Publication No. (SMA) 04-3939. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004. [PubMed]
- 17.Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs, Treatment Improvement Protocol (TIP) Series 43. DHHS Publication No. (SMA) 05-4048. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2005. [PubMed]
- 18.Burt MR, Aron LY, Douglas T, Valente J, Lee E, and Iwen B. Homelessness: programs and the people they serve. Technical report of findings of the National Survey of Homeless Assistance Providers and Clients. 1999. Interagency Council on the Homeless.
- 19.Fischer PJ and Breakey WR. The epidemiology of alcohol, drug, and mental disorders among homeless persons. Am J Psychol. 1991;46:1115–28. [DOI] [PubMed]
- 20.Stahler GJ and Cohen E. Homelessness and substance abuse in the 1990s. Contemp Drug Probl. 1995;22:169–92.
- 21.Koegel P, Sullivan G, Burnam A, Morton SC, and Wenzel S. Utilization of mental health and substance abuse services among homeless adults in Los Angeles. Med Care. 1999;37:306–17. [DOI] [PubMed]
- 22.Hibbs JR, Benner L, Klugman L, et al. Mortality in a cohort of homeless adults in Philadelphia. N Engl J Med. 1994;331:304–9. [DOI] [PubMed]
- 23.Hwang SW, Lebow JM, Bierer MF, O’Connell JJ, Orav J, and Brennan TA. Risk factors for death in homeless adults in Boston. Arch Int Med. 1998;158:1454–60. [DOI] [PubMed]
- 24.Wright JD and Weber E. Homelessness and health. New York, NY: McGraw-Hill, Inc; 1987.
- 25.Seal KH, Kral AH, Gee L, et al. Predictors and prevention of nonfatal overdose among street-recruited injection heroin users in the San Francisco Bay Area, 1998–1999. Am J Public Health. 2001;91:1842–6. [DOI] [PMC free article] [PubMed]
- 26.Blanchon T, Boissonnas A, Vareseon I, and Vidal-Trecan G. Homelessness and high-dosage buprenorphine misuse. Subst Use Misuse. 2003;38:429–42. [DOI] [PubMed]
- 27.Thomas L, Kelly M, and Cousineau M. Alcoholism and substance abuse. In: Brickner PW, Scharer LK, Conanan BA, Savarese M, and Scanlan BC, eds. Under the safety net: The health and social welfare of the homeless in the United States. New York, NY: W.W. Norton & Co.; 1990;204–14.
- 28.Song JY, Safaeian M, Strathdee SA, Vlahov D, and Celentano DD. The prevalence of homelessness among injection drug users with and without HIV infection. J Urban Health. 2000;77:678–87. [DOI] [PMC free article] [PubMed]
- 29.Galanter M, Dermatis H, Glickman L, et al. Network therapy: decreased secondary opioid use during buprenorphine maintenance. J Subst Abuse Treat. 2004;26:313–8. [DOI] [PubMed]
- 30.Kertesz SG, Horton NJ, Friedmann PD, Saitz R, and Samet JH. Slowing the revolving door: stabilization programs reduce homeless persons’ substance use after detoxification. J Subst Abuse Treat. 2003;24:197–207. [DOI] [PubMed]
- 31.Milby JB, Schumacher JE, Raczynski JM, et al. Sufficient conditions for effective treatment of substance abusing homeless persons. Drug Alcohol Depend. 1996;43:39–47. [DOI] [PubMed]
- 32.Shah NG, Celentano DD, Vlahov D, et al. Correlates of enrollment in methadone maintenance treatment programs differ by HIV-serostatus. AIDS. 2000;14:2035–43. [DOI] [PubMed]
- 33.Mistral W and Velleman R. Are practice nurses an underused resource for managing patients having problems with illicit drugs? J Subst Use. 1999;4:82–7. [DOI]
- 34.SAS Institute Inc. SAS/STAT Software: Changes and Enhancements, Release 8.2. Cary, NC: SAS Institute Inc.; 2001.
- 35.Berwick DM. A user’s manual for the IOM’s ‘quality chasm’ report. Health Aff. 2002;21:80–90. [DOI] [PubMed]


