Abstract
BACKGROUND
Soft tissue infections (STIs) from injection drug use are a common cause of Emergency Department visits, hospitalizations, and operating room procedures, yet little is known about factors that may predict the need for these costly medical services.
OBJECTIVE
To describe a cohort of injection drug users seeking Emergency Department care for STIs and to identify risk factors associated with hospitalization. We hypothesized that participants who delayed seeking care would be hospitalized more often than those who did not.
DESIGN
Cohort study using in-person structured interviews and medical record review. Logistic regression assessed the association between hospital admission and delay in seeking care as well as other demographic, clinical, and psychosocial factors.
PARTICIPANTS
Injection drug users who sought Emergency Department care for STIs from May 2001 to March 2002.
RESULTS
Of the 136 participants, 55 (40%) were admitted to the hospital. Delay in seeking care was not associated with hospital admission. Participants admitted for their infection were significantly more likely to be living in a shelter (P = .01) and to report being hospitalized 2 or more times in the past year (P < .01).
CONCLUSIONS
We identified a subpopulation of injection drug users, mostly living in shelters, who were hospitalized frequently in the past year and who were more likely to be hospitalized for their current infections compared to others. As members of this subpopulation can be easily identified and located, they may benefit from interventions to reduce the health care utilization resulting from these infections.
KEY WORDS: injection drug use, soft tissue infection, cellulitis, abscess, substance abuse
BACKGROUND
Injection drug use has many well-recognized adverse effects on health including overdose, withdrawal, viral hepatitis, HIV, endocarditis, and infections of skin and soft tissues. Soft tissue infections (STIs) are particularly common, affecting up to 32% of injection drug users at a given time.1 These infections are painful and costly to patients and may progress to more serious life- and limb-threatening infections.2,3 The growing epidemic of methicillin-resistant Staphylococcus aureus STIs, particularly among injection drug users, has complicated treatment of these infections.4–7
For urban hospitals that serve a large number of uninsured or publicly insured patients, injection drug use–related STIs consume significant resources in Emergency Departments, inpatient wards, and operating rooms. For example, STIs accounted for more than 3,000 Emergency Department visits to San Francisco General Hospital in 1999 and were the most common reason for nonpsychiatric hospitalization. Abscess incision and drainage was the most common primary procedure for all inpatients.8 Based on medical record reviews, 70% of the infections resulted from injection drug use, and annual Emergency Department and inpatient costs of treating the infections were estimated at 20 million dollars.8 Similarly, at Detroit Receiving Hospital more than 20 years ago, inpatient costs for the care of injection drug use–related abscesses alone were 6.9 million dollars for 1 year.9 A community-recruited cohort of 598 injection drug users in Vancouver, British Columbia experienced 2,763 Emergency Department visits and 495 admissions to 1 hospital in a 40-month period, and STI was the most common admission and Emergency Department diagnosis for those patients.10 A medical record review conducted at the same hospital as the present study documented that approximately 39% of injection drug users who sought care for STIs in the Emergency Department were hospitalized for treatment of their infections.11 Despite this research showing the burden of injection drug use–related STIs, no study has evaluated potentially modifiable risk factors that might be associated with more severe STIs that require hospitalization.
We sought to describe a cohort of injection drug users seeking Emergency Department care for STIs at an urban, public hospital in Washington State and to identify modifiable risk factors associated with hospitalization. In particular, we hypothesized a priori that participants who delayed seeking care for their STIs would be hospitalized more often than those who did not delay seeking care. We also sought to identify any other patient-related or environmental barriers to care that were associated with a delay in seeking care and subsequent need for hospitalization (Fig. 1). For participants with abscesses, we assessed the association between these barriers to care and need for operating room incision and drainage.
Figure 1.
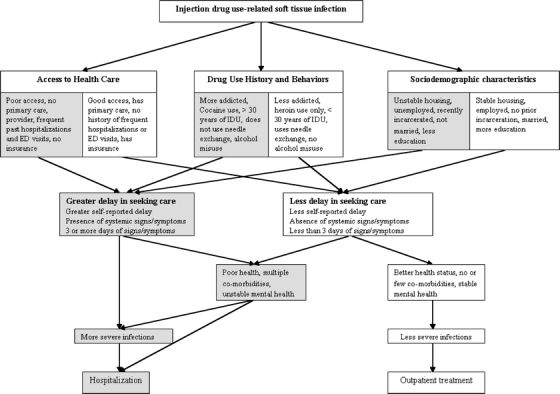
Hypothesized relationships between barriers to seeking care, delay in seeking care, soft tissue infection severity, and Emergency Department (ED) disposition.
METHODS
Study Design
This cohort study used in-person interviews and hospital records of injection drug users seeking Emergency Department care for STIs. The institutional review board approved study procedures, including written informed consent and acquisition of a certificate of confidentiality from the National Institutes of Health.
Setting
Recruitment of participants was conducted at an urban, county hospital Emergency Department in Washington State, from May 2001 to March 2002. Participants were interviewed during their Emergency Department visit when possible. However, some interviews were completed after hospital admission or discharge from the Emergency Department.
Participants
Eligible patients were English-speaking injection drug users who were seeking initial care for a STI and who agreed to a 45-minute interview and review of their hospital records. Emergency Department providers identified potential subjects and assessed their willingness and ability to participate in the study. Four trained research assistants spent an average of 59 hours per week interviewing participants between 8 a.m. and 10 p.m. Based on a previously published medical record review that identified 242 injection drug users seeking care for an STI at the same Emergency Department during a 26-week period in 1999–2000, we estimated that approximately 409 injection drug users would seek Emergency Department care for STIs during the 44-week interview period, with most patients presenting between 8 a.m. and 10 p.m..11 In the present study, Emergency Department staff identified 211 eligible patients who were able and agreed to speak to a research assistant and of those, 156 (74%) agreed to participate and completed the interview. Those who completed the interview were remunerated with a $20 gift certificate for groceries. We excluded 20 participants who left the Emergency Department against medical advice or before completion of their medical evaluation, reducing the study population to 136 patients.
Data Collection
We developed a semistructured interview instrument to specifically assess whether participants delayed seeking medical care, our hypothesized predictor of hospitalization. In addition, the interview sought information about factors hypothesized to affect STI severity or present barriers to seeking medical care, resulting in a delay in seeking care. These questions spanned 5 domains: (1) illicit drug use and behaviors (2) current STI characteristics, (3) health care utilization and access to care, (4) general health, medical comorbidity and psychiatric symptoms, and (5) sociodemographic characteristics. Previously used measures were incorporated when available (described below). Interviewers utilized calendars and other visual prompts shown previously to improve recall reliability in patients with substance abuse problems.12
In addition to interview data, the hospital electronic medical record system was accessed to obtain information on patients’ Emergency Department treatment, insurance status, surgical procedures, and hospitalizations.
Measures
Hospital Utilization
Hospital admission for an STI was selected as this study’s a priori primary outcome, because it reflects STI severity as well as the burden of the STI on the patient and society. Whether or not patients were hospitalized was obtained from hospital records. For patients with abscesses, a secondary outcome of whether incision and drainage was performed in the operating room versus at the bedside in the Emergency Department or hospital room was also obtained from hospital records.
Delay in Seeking Care
Several measures of delay in seeking care were assessed. First, participants were asked to explicitly report whether they had delayed seeking medical care for their current STIs, and if so, for how many days (0, 1–3, 4–7, or more than 7). Second, participants were asked about the timing of signs and symptoms of infection as another means of assessing potential delay in seeking care. Specifically, they were asked if they had any systemic (fevers, high temperature, or sore muscles) or local (redness, warmth, swelling, drainage, or pain) signs or symptoms of infection. Those who reported any signs or symptoms of infection were then asked to estimate the number of days they had had each sign or symptom (0, 1–3, 4–7, or more than 7).
Access to Health Care
To assess potential barriers to care, participants were asked about their previous experiences with the health care system, including whether they had a primary care provider and the number of times they were hospitalized in the past year. Participants’ insurance or health benefits were ascertained from hospital records.
Drug Use History and Behaviors
Drug use history and behaviors were assessed using questions from previous epidemiologic studies of illicit drug users whenever possible.13,14 Participants were asked about their last drug injection, including the type of drug they injected (heroin, cocaine, heroin and cocaine, amphetamines), whether they injected into a vein, subcutaneously, intramuscularly, or missed a vein, and their skin and syringe-cleaning behaviors. Number of years of injection drug use (0–9, 10–19, 20–29, or 30–41), the drug(s) participants injected most frequently, and their skin, syringe, and needle-cleaning behaviors were also ascertained from interviews. The AUDIT-C alcohol screening questionnaire was used to assess alcohol misuse, including risky drinking and alcohol use disorders.15 An AUDIT-C score of 4 or more was considered a positive screen for alcohol misuse.16
Soft Tissue Infection Characteristics
The type, location, and number of current STIs were determined by review of Emergency Department documentation and interviews. STIs were categorized as being abscesses, cellulitis, or infected ulcers. Infections were considered abscesses if an incision and drainage procedure yielded purulent material or if spontaneous drainage of an infection was reported by the patient or in provider notes. STI locations were grouped according to the following anatomic sites: shoulder, arm, leg, buttock, 2 or more sites, and other sites. Participants were asked to estimate the number of previous STIs they had experienced and to describe prior self-treatment of their current infection, including use of antibiotics and incision and drainage attempts.
General Health, Medical Comorbidity and Psychiatric Symptoms
Participants’ overall health status (poor, fair, good, very good, excellent) was based on self-report. Psychiatric symptoms were assessed using the psychiatric status section of the Addiction Severity Index (ASI). Psychiatric status composite scores were calculated using the formulas supplied by the ASI Composite Scores Manual17 and could range from 0 to 1 with higher scores indicating a higher “severity rating.”
Sociodemographic Characteristics
During the interview, participants were asked about their current living situation (own or rent a house or apartment; someone else’s house or apartment; hotel, motel, boarding house, halfway house; shelter; on the streets, car, or abandoned building; other) and whether they considered themselves homeless. They were asked about their level of education, employment status, and whether they had ever been incarcerated.
Statistical Analyses
Initially, the relationship between measures of delay in seeking care and the study’s primary outcome, hospitalization, was assessed with bivariate logistic regression. Secondary exploratory bivariate analyses using logistic regression explored whether study participants’ drug use history and behaviors, STI characteristics, previous health care utilization, medical comorbidity, psychiatric symptoms, and sociodemographic characteristics were associated with hospital admission. We then constructed a multivariable logistic regression model that included all factors significantly associated with hospitalization on bivariate analyses to determine their independent relationship with hospitalization. Associations with p values <.05 were considered statistically significant.
Similarly, for participants who had injection drug use–related abscesses, we used bivariate logistic regression to assess the relationship between need for operating room incision and drainage and measures of delay in seeking care. Exploratory bivariate analyses were used to explore whether particular drug use history and behaviors, STI characteristics, previous health care utilization, medical comorbidity and psychiatric symptoms, and sociodemographic characteristics predicted operating room incision and drainage. Factors that were significantly associated with operating room incision and drainage on bivariate analyses (P < .05) were included in a multivariable logistic regression model to test their independent association with operating room incision and drainage.
Statistical analyses were performed using STATA statistical software (STATA Corp, College Station, TX).
RESULTS
A total of 136 patients who sought initial care for 147 soft tissue infections in the Emergency Department consented to participate and completed the in-person interview and their medical evaluation (Table 1). Although more than 75% of participants reported that they graduated from high school or obtained their general educational development (GED) credential , only 11% reported having a job and nearly one-third lived on the streets or in shelters. The 136 study participants had a total of 147 infections and the characteristics of their infections are presented in Table 2.
Table 1.
Study Population Characteristics
| Demographics | Discharged from ED | Hospitalized | Total Sample | P value |
|---|---|---|---|---|
| N = 81 N (%) | N = 55 N (%) | N = 136* N (%) | ||
| Mean age, (SD), yrs | 43 (8) | 41 (8) | 43 (8) | .19 |
| Female | 28 (35) | 24 (44) | 52 (38) | .22 |
| Non-Hispanic White | 49 (60) | 36 (65) | 85 (63) | .52 |
| Living situation | .05 | |||
| Owns/rents home | 28 (35) | 13 (24) | 41 (30) | |
| Street | 15 (19) | 11 (20) | 26 (19) | |
| Living in a shelter | 5 (6) | 13 (24) | 18 (13) | |
| Other† | 33 (41) | 18 (33) | 51 (38) | |
| Graduated from high school/GED‡ | 62 (77) | 42 (76) | 104 (76) | .97 |
| Currently employed | 9 (11) | 6 (11) | 15 (11) | 1.00 |
| Reported time in jail, prison, or juvenile detention | 71 (88) | 41 (75) | 112 (82) | .18 |
| Health care utilization and access to care | ||||
| Two or more hospitalzations past year | 7 (9) | 18 (33) | 25 (18) | <.01 |
| Insurance benefits | .96 | |||
| Medicare/Medicaid | 40 (49) | 39 (71) | 79 (58) | |
| Self Pay | 13 (16) | 13 (24) | 29 (19) | |
| Other/Unknown | 28 (35) | 3 (5) | 31 (23) | |
| Has a primary care provider | 37 (46) | 19 (35) | 56 (41) | .26 |
| Delay in seeking care | ||||
| Considered coming in for the infection before today | 63 (78) | 43 (78) | 106 (78) | .80 |
| One or more days of ANY symptoms | 59 (73) | 42 (76) | 101 (74) | .30 |
| One or more days of SYSTEMIC§ symptoms | 64 (79) | 50 (91) | 114 (84) | .30 |
| Drug use history and behaviors | ||||
| Reported 10 or more years of injection drug use | 59 (73) | 40 (73)|| | 99 (73)|| | .99 |
| Last injection site | .66 | |||
| Vein | 7 (9) | 5 (9) | 12 (9) | |
| Muscle | 32 (40) | 22 (40) | 54 (40) | |
| Other | 42 (52) | 28 (51) | 70 (51) | |
| Most frequently injected drug is heroin only | 68 (84) | 51 (93) | 119 (88) | .07 |
| Reported using a needle exchange program | 70 (86) | 51 (93) | 121 (89) | .14 |
| Did not clean skin before last injection | 33 (41) | 23 (42) | 56 (41) | .90 |
| Hazardous drinking (AUDIT-C score >4) | 25 (31) | 13 (24) | 38 (28) | .34 |
| General health, medical comorbidity, and psychiatric symptoms | ||||
| My health is poor | 17 (21) | 14 (25) | 31 (23) | .51 |
| Median ASI Psychiatric Status composite score (range) | 0.5 (0–1) | 0.4 (0–0.8) | 0.4 (0–1) | .08 |
*Excludes subjects with missing data and 20 subjects who left the ED against medical advice or prior to medical evaluation.
†Someone else’s house or apartment, hotel/motel, boarding house, half-way house.
‡General educational development credential.
§Fevers, chills, muscle aches.
||Percentages do not sum to 100% due to missing values.
Table 2.
Current Soft Tissue Infection Characteristics
| Discharged from ED N = 81 | Hospitalized N = 55 | Total Sample N = 136* | P value | |
|---|---|---|---|---|
| Type of soft tissue infection | .79 | |||
| Abscess | 58 (72) | 43 (78) | 101 (74) | |
| Only cellulites | 15 (19) | 8 (15) | 23 (17) | |
| Infected ulcer/unknown | 8 (10) | 4 (6) | 12 (9) | |
| Location of soft tissue infection | .74 | |||
| Shoulder region | 12 (15) | 9 (16) | 21 (15) | |
| Arm (excluding shoulder region) | 30 (37) | 15 (27) | 45 (33) | |
| Leg | 17 (21) | 11 (20) | 28 (21) | |
| Buttock | 11 (14) | 11 (20) | 22 (16) | |
| Other locations/multiple sites | 11 (14) | 9 (16) | 20 (15) | |
| More than 1 current STI | 42 (52) | 27 (49) | 69 (51) | .75 |
| Self-treatment with oral antibiotics | 21 (26) | 14 (25) | 35 (26) | 1.00 |
| Self-treatment with incision and drainage | 33 (41) | 22 (40) | 55 (40) | 1.00 |
* Excludes subjects with missing data and 20 subjects who left the ED against medical advice prior to medical evaluation.
Of the 136 participants, 55 (40%) were admitted to the hospital. Contrary to our hypothesis, this study’s measures of delay in seeking care were not associated with hospitalization. Patients who were admitted to the hospital did differ in their previous health care utilization and current living situation compared to those who were not admitted. Specifically, participants who were hospitalized for treatment of their STIs were significantly more likely to report being hospitalized 2 or more times in the past year and to be living in a shelter compared to those treated as outpatients (Table 3). The type and location of infection and other demographic characteristics presented in Table 1 were not associated with hospitalization.
Table 3.
Predictors of Hospitalization for Sti from the Emergency Department
| Odds Ratio (95% Confidence Interval) | ||
|---|---|---|
| Bivariate | Multivariate | |
| Living situation | ||
| Owns or rents a home | Reference | Reference |
| Lives in a shelter | 5.6 (1.6–19.0) | 4.2 (1.2–15.1) |
| Lives on the streets | 1.6 (0.6–4.4) | 1.4 (0.5–4.1) |
| Other* | 1.0 (0.4–2.5) | 1.1 (0.5–2.8) |
| Hospitalized 2 or more times in the past year | ||
| No | Reference | Reference |
| Yes | 2.7 (0.9–7.7) | 4.4 (1.6–11.8) |
*Someone else’s house or apartment, hotel/motel, boarding house, half-way house.
Frequently Hospitalized Patients
The subgroup of 25 participants who reported being hospitalized 2 or more times in the past year comprised 18% of the overall study population and 33% of those hospitalized for treatment of their STIs. Whereas 72% of this subgroup was admitted for their current infection, only 33% of the rest of the study population was admitted. No participant in this subgroup reported being employed. Based on exploratory logistic regression analyses, living in a shelter and injecting drugs for 30 years or more were independently associated with being hospitalized 2 or more times in the past year (Table 4). The type and location of infection and other demographic characteristics presented in Table 1 were not associated with being hospitalized 2 or more times in the past year.
Table 4.
Predictors of being Hospitalized Two or More Times in the Past Year
| Odds Ratio (95% Confidence Interval) | ||
|---|---|---|
| Bivariate | Multivariate | |
| Reported current living situation | ||
| Owns/rents a home | Reference | Reference |
| Lives in a shelter | 4.7 (1.3–16.6) | 4.5 (1.1–19.2) |
| Lives on the streets | 1.8 (0.5–6.2) | 2.2 (0.6–8.7) |
| Other* | 0.7 (0.2–2.4) | 0.96 (0.2–3.5) |
| Reported years of injection drug use | ||
| 0–9 | Reference | Reference |
| 1–19 | 0.7 (0.2–3.3) | 0.7 (0.1–3.9) |
| 20–29 | 1.4 (0.3–5.9) | 1.4 (0.3–6.3) |
| 30–41 | 5.2 (1.4–18.4) | 4.3 (1.1–16.8) |
| Reported chronic medical problems | 2.6 (1.0–6.6) | 2.2 (0.8–6.3) |
| More than 1 current soft tissue infection | 2.5 (1.0–6.2) | 2.6 (0.9–7.4) |
*Someone else’s house or apartment, hotel/motel, boarding house, half-way house.
Patients with Abscesses
Among the 101 (69%) participants with abscesses, 60% had their infection incised and drained at the bedside, whereas 23% were incised and drained in the operating room and 3% had incision and drainage procedures both at the bedside and in the operating room. Ten percent had abscesses that had drained previously, either spontaneously or by self-incision and drainage and 4% of participants had suspected abscesses that were not drained. On unadjusted analyses, participants who reported having systemic symptoms for more than 7 days were more likely to undergo operating room incision and drainage compared to those reporting no systemic symptoms (odds ratio [OR] 6.0, 95% CI 1.1–33.3) and participants with abscesses in the shoulder region were more likely to undergo operating room incision and drainage compared to those with abscesses in other areas (OR 3.0, 95% CI 1.1–8.1). In a multivariable model, only the location of the abscess in the shoulder region independently predicted operating room incision and drainage (OR 3.1, 95% CI 1.1–8.9).
DISCUSSION
Many injection drug users with STIs seek care in emergency departments [10], and many are subsequently hospitalized [11]. We hypothesized that a delay in seeking care might be a modifiable risk factor for hospital admission and more severe abscesses requiring operating room incision and drainage. This study’s measures of delay in seeking care included participants’ self-report of delay, their estimated duration of delay, and the duration of any signs and symptoms of infection. Contrary to our hypothesis, none of these measures of delay were associated with hospitalization or operating room incision and drainage.
This study also prospectively evaluated the association of patient characteristics, including potentially modifiable risk factors, with hospital admission for an injection drug use–related STI. Only living in a shelter and multiple hospitalizations in the past year were significantly associated with hospitalization. Frequent hospitalizations for STIs were in turn associated with years of injection drug use. These findings highlight a group of patients with repeated hospitalizations for STIs who, when hospitalized, would be easily accessible to counselors and targeted interventions.
This study’s finding that injection drug users who lived in a shelter were 4 times more likely to be hospitalized suggests that outreach to shelters might impact hospital utilization by this population. However, the mechanism accounting for this finding is uncertain. Unstable housing may influence a physician’s decision to admit a patient, and patients who reside in shelters may be more willing to be admitted than those with homes. Specific characteristics of shelters may also impact the severity of an infection and need for hospitalization. For example, the close quarters of shelters might foster more virulent or antibiotic-resistant STI pathogens.5
Abscess incision and drainage in the operating room rather than at the bedside was another measure of hospital utilization in this study. Nearly a quarter of participants with abscesses underwent operating room incision and drainage and the location of an abscess in the shoulder region was the only independent predictor of operating room incision and drainage. Shoulder injections are primarily intramuscular and thus related infections may be deeper and require more extensive incision and drainage than can be managed at the bedside.
Previous studies have identified drug use behaviors associated with developing STIs. A community-based study of injection drug users found that not cleaning the skin before injection, subcutaneous or intramuscular injection, and injecting a combination of heroin and cocaine increased the risk of developing an abscess.18 However, in the present study, these practices were not associated with hospitalization or operating room incision and drainage. Likewise, previous studies have suggested that recent incarceration might increase carriage of resistant organisms19,20 resulting in increased hospitalizations, but we did not find such an association. Thus, risk factors for STI development and risk factors for health care utilization among patients seeking care for STIs in public hospitals appear to be distinct.
Several limitations of this study are noteworthy. It included only patients who sought care for a STI at 1 hospital’s Emergency Department and not patients evaluated elsewhere. The lack of an association between delay in seeking care and hospitalization in this study may be caused by the fact that patients with STIs who truly did not delay care were seen outside the Emergency Department. In addition, the relationship between symptom severity, rapidity of symptom development, and the decision to seek care is likely complex and may not relate to hospitalization in a consistent manner. We excluded 20 participants who left the Emergency Department against medical advice or before completion of their medical evaluation because we were not able to ascertain their true Emergency Department disposition. If those participants delayed seeking care and should have been hospitalized, our primary hypothesis could have been supported. Because we were not able to collect information from eligible patients who declined or were not asked to participate, we could not assess the impact of any recruitment bias. We did not collect specific information regarding participants’ chronic medical illnesses, including HIV disease, which might influence infection severity and need for hospitalization. Lastly, the study may have lacked the power to detect associations between hospitalization or operating room procedures and other factors owing to small sample size. Its findings should be considered preliminary and exploratory as no adjustment was made for multiple comparisons.
Urban public hospitals and their Emergency Departments bear a disproportionate burden in caring for patients with complications related to substance abuse and would benefit most from studies aimed at reducing utilization by this population. We found that injection drug users who lived in shelters and who had been injecting for more than 30 years were more likely to have been hospitalized 2 or more times in the past year. These data could help target strategies to reduce the health care utilization resulting from these infections, and reduce the pain and suffering of this marginalized population.
Acknowledgment
This research was supported by the Health Services Research and Development Service, Department of Veterans Affairs; University of Washington Alcohol and Drug Abuse Institute, and the Robert Wood Johnson Foundation Clinical Scholars Program. Dr. Takahashi is a staff physician at the VA Puget Sound Health Care System and is currently supported by the National Institute on Drug Abuse (NIDA #RO3DA14518). Dr. Baernstein is a staff physician at Harborview Medical Center. Dr. Binswanger is an investigator at University of Colorado and was a VA Fellow in the Robert Wood Johnson Clinical Scholars Program at the University of Washington at the time this study was conducted. Dr. Bradley is an investigator at the VA Puget Sound Health Care System and is currently supported by the National Institute of Alcohol Abuse and Alcoholism (NIAAA #K23AA00313) and was a Robert Wood Johnson Foundation Generalist Physician Faculty Scholar at the time this study was conducted. Dr. Merrill has received funding from Harborview Medical Center in support of this work.
Potential Financial Conflicts of Interest None disclosed.
Footnotes
This study was presented as a poster at the Society of General Internal Medicine 27th Annual Meeting, Chicago, Illinois, May 2004.
The views expressed in this article are those of the authors and do not necessarily represent the views of the Robert Wood Johnson Foundation, Department of Veterans Affairs, University of Washington or University of Colorado.
References
- 1.Binswanger IA, Kral AH, Bluthenthal RN, Rybold DJ, Edlin BR. High prevalence of abscesses and cellulitis among community-recruited injection drug users in San Francisco. Clin Infect Dis. 2000;30(3):579–81. [DOI] [PubMed]
- 2.CDC. Unexplained illness and death among injecting-drug users—Glasgow, Scotland; Dublin, Ireland; and England, April–June 2000. MMWR. 2000;49(22):489–92. [PubMed]
- 3.Lonergan S, Rodriguez RM, Schaulis M, Navaran P. A case series of patients with black tar heroin-associated necrotizing fasciitis. J Emerg Med. 2004;26(1):47–50. [DOI] [PubMed]
- 4.Charlebois ED, Bangsberg DR, Moss NJ, et al. Population-based community prevalence of methicillin-resistant Staphylococcus aureus in the urban poor of San Francisco [see comment]. Clin Infect Dis. 2002;34(4):425–33. [DOI] [PubMed]
- 5.Frazee BW, Lynn J, Charlebois ED, Lambert L, Lowery D, Perdreau-Remington F. High prevalence of methicillin-resistant Staphylococcus aureus in Emergency Department skin and soft tissue infections. Ann Emerg Med. 2005;45(3):311–20. [DOI] [PubMed]
- 6.Young DM, Harris HW, Charlebois ED, et al. An epidemic of methicillin-resistant Staphylococcus aureus soft tissue infections among medically underserved patients. Arch Surg. 2004;139(9):947–51; discussion 951–3. [DOI] [PubMed]
- 7.Morgan G, Krishnadasan A, Gorwitz R, et al. Methicillin-resistant S. aureus infections among patients in the Emergency Department. N Engl J Med. 2006;355(7):666–74. [DOI] [PubMed]
- 8.CDC. Soft tissue infections among injection drug users—San Francisco, California, 1996–2000. MMWR. 2001;50(19):381–4. [PubMed]
- 9.Wallace JR, Lucas CE, Ledgerwood AM. Social, economic, and surgical anatomy of a drug-related abscess. Am Surgeon. 1986;52(7):398–401. [PubMed]
- 10.Palepu A, Tyndall MW, Leon H, et al. Hospital utilization and costs in a cohort of injection drug users. CMAJ. 2001;165(4):415–20. [PMC free article] [PubMed]
- 11.Takahashi TA, Merrill JO, Boyko EJ, Bradley KA. Type and location of injection drug use-related soft tissue infections predict hospitalization. J Urban Health. 2003;80(1):127–36. [DOI] [PMC free article] [PubMed]
- 12.Sacks JA, Drake RE, Williams VF, Banks SM, Herrell JM. Utility of the time-line follow-back to assess substance use among homeless adults. J Nerv Ment Dis. 2003;191(3):145–53. [DOI] [PubMed]
- 13.Hagan H, McGough JP, Thiede H, Hopkins S, Duchin J, Alexander ER. Reduced injection frequency and increased entry and retention in drug treatment associated with needle-exchange participation in Seattle drug injectors. J Subst Abuse Treat. 2000;19(3):247–52. [DOI] [PubMed]
- 14.Kral AH, Bluthenthal RN, Lorvick J, Gee L, Bacchetti P, Edlin BR. Sexual transmission of HIV-1 among injection drug users in San Francisco, USA: risk-factor analysis. Lancet. 2001;357(9266):1397–401. [DOI] [PubMed]
- 15.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA, for the Ambulatory Care Quality Improvement Project. The AUDIT Alcohol Consumption Questions (AUDIT-C): an effective brief screening test for problem drinking. Arch Intern Med. 1998;158(16):1789–95. [DOI] [PubMed]
- 16.Dawson DA, Grant BF, Stinson FS, Zhou Y. Effectiveness of the derived Alcohol Use Disorders Identification Test (AUDIT-C) in screening for alcohol use disorders and risk drinking in the US general population. Alcohol Clin Exp Res. 2005;29(5):844–54. [DOI] [PubMed]
- 17.McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the addiction severity index. J Subst Abuse Treat. 1992;9(3):199–213. [DOI] [PubMed]
- 18.Murphy EL, DeVita D, Liu H, et al. Risk factors for skin and soft-tissue abscesses among injection drug users: a case-control study. Clin Infect Dis. 2001;33(1):35–40. [DOI] [PubMed]
- 19.Weber JT. Community-associated methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2005;41(S4):S269–72. [DOI] [PubMed]
- 20.Baillargeon J, Kelley MF, Leach CT, Baillargeon G, Pollock BH. Methicillin-resistant Staphylococcus aureus infection in the Texas prison system. Clin Infect Dis. 2004;38(9):e92–5. [DOI] [PubMed]


