Abstract
Background
Several guidelines recommend initiating colorectal cancer screening at age 40 for individuals with affected first-degree relatives, yet little evidence exists describing how often these individuals receive screening procedures.
Objectives
To determine the proportion of individuals in whom early initiation of colorectal cancer screening might be indicated and whether screening disparities exist.
Design
Population-based Supplemental Cancer Control Module to the 2000 National Health Interview Survey.
Participants
Respondents, 5,564, aged 40 to 49 years were included within the analysis.
Measurements
Patient self-report of sigmoidoscopy, colonoscopy, or fecal occult blood test.
Results
Overall, 279 respondents (5.4%: 95% C.I., 4.7, 6.2) reported having a first-degree relative affected with colorectal cancer. For individuals with a positive family history, 67 whites (27.9%: 95% C.I., 21.1, 34.5) and 3 African American (9.3%: 95% C.I., 1.7, 37.9) had undergone an endoscopic procedure within the previous 10 years (P-value = .03). After adjusting for age, family history, gender, educational level, insurance status, and usual source of care, whites were more likely to be current with early initiation endoscopic screening recommendations than African Americans (OR = 1.38: 95% C.I., 1.01, 1.87). Having an affected first-degree relative with colorectal cancer appeared to have a stronger impact on endoscopic screening for whites (OR = 3.21: 95% C.I., 2.31, 4.46) than for African Americans (OR = 1.05: 95% C.I., 0.15, 7.21).
Conclusions
White participants with a family history are more likely to have endoscopic procedures beginning before age 50 than African Americans.
Key words: colorectal cancer, family history, screening, prevention, health disparities, primary care
BACKGROUND
Randomized controlled trials and observational studies have demonstrated that colorectal cancer screening with fecal occult blood testing (FOBT) and/or endoscopy reduces cancer mortality and incidence.1–6 Indeed, the use of these screening procedures is believed to have contributed to the decline seen in colorectal cancer (CRC) mortality over the last few decades.7 In the general population, the incidence of CRC begins to increase steeply with age with over 90% of cases occurring after 50 years of age.8 As such, most organizations recommend initiating CRC screening beginning at age 50 for average risk people.9–11
For individuals who are at an increased risk of developing CRC because of their family history, the most effective screening strategy remains to be fully elucidated.12 Several studies have confirmed that having an affected relative with CRC can double to quadruple one’s lifetime risk of developing colorectal cancer.13 Incident cancer cases in individuals with affected first-degree relatives present, on average, 10 years earlier than in individuals with no affected relatives.14 This “age shift” in CRC incidence rates underlies much of the rationale for initiating screening at age 40 in individuals with affected first-degree relatives.9
Although overall mortality rates for CRC have been on the decline, the rate of this decline has been greater for whites than African Americans.7 African Americans have a 19% higher incidence rate of CRC and a 39% higher mortality rate.15 Part of the reason for this mortality difference results from African Americans being more likely to present with advanced stages of CRC.16,17 Another factor that may contribute to higher CRC mortality rates in African Americans might be inequities in screening rates. Although screening inequities have been described for average risk individuals, they have typically been modest and more often related to socioeconomic status than race.18–23 Nevertheless, even modest differences in screening rates could be related to survival differences if the individuals with the greatest risk for developing CRC are less likely to undergo screening. The purpose of this study was to determine the prevalence of individuals who might be candidates for early initiation of CRC screening based on their family history and to determine whether screening rates differ between African Americans and white individuals with affected first-degree relatives.
DESIGN, PARTICIPANTS, MEASUREMENTS
The data source for this study was the Cancer Control Module of the 2000 National Health Interview Survey (NHIS). The NHIS is an in-person health interview conducted by the U.S. Census Bureau for the National Center for Health Statistics.24 The NHIS uses a multistage probability sample design to represent current health information for the civilian, noninstitutionalized, household population of the United States. For the year 2000 survey, the overall sample adult response rate was 72.1%.25 In 2000, a supplement to the core NHIS was the Cancer Control Module.24 This module consisted of seven sections covering health behavior, family cancer history, and cancer screening. For the 2000 Cancer Control Module, 32,374 persons, 18 years or older, were interviewed. A total of 5,564 respondents were eligible for our analysis (Fig. 1).
Figure 1.
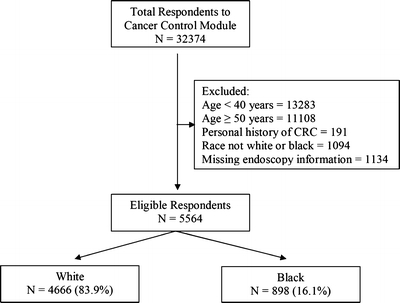
Study population CRC = colorectal cancer.
All adults over the age of 40 were asked about CRC screening procedures and the date of the most recent examination. A respondent was considered to have undergone CRC screening if they reported ever having had a colonoscopy, sigmoidoscopy, or home-based fecal occult blood testing (FOBT). Because office-based fecal occult testing is not considered an appropriate CRC screening strategy, we did not include this within our analysis.9 The 2000 Cancer Control Module did not inquire about barium enema testing, and as such, this variable could not be included within our analysis.
We considered respondents to be current with FOBT if they reported having at least one test within the past year. Sigmoidoscopy and colonoscopy were collapsed into a single endoscopy variable. Respondents were considered to be current with endoscopic screening if they reported having at least one of these procedures within the previous 10 years. Because sigmoidoscopy screening is recommended to occur at 5-year intervals, we performed a secondary analysis in which we defined being current with endoscopic screening as reporting at least one procedure during the previous 5 years. Models were then constructed using both definitions (10 and 5 years) for endoscopic screening. The primary analysis included the 10-year screening definition. When data are presented relating to the 5-year interval definition, this is indicated specifically within the text.
To determine if an individual might be a candidate for early initiation of cancer screening, we used guidelines available in 2000.26–28 At this time, the American Cancer Society was recommended initiating CRC screening at age 40 in individuals with either a first-degree relative affected with CRC before age 60 years while the American Gastroenterology Association (AGA) suggested early initiation of CRC surveillance for any individual with an affected first-degree relative.27 For the purpose of this analysis, we used the conservative AGA recommendations and considered any individuals with an affected first-degree relative, regardless of age, as a candidate for early initiation of CRC screening.
Respondents who reported undergoing endoscopy or FOBT were asked to give the reason for undergoing the procedure. Responses were categorized as: (1) part of a routine physical exam/screening test, (2) because of a specific problem, (3) follow-up test of an earlier test or screening exam, (4) family history, or (5) other. Respondents also rated their own perceived likelihood of developing cancer by responding to the question, “Would you say your risk of getting cancer in the future is low, medium, or high.” For the purpose of our analysis, we dichotomized this response into “low likelihood” and “medium or high likelihood.”
We used self-reported demographic information to construct our independent covariates. Individuals who reported their typical source of health care as a clinic, health center, doctor’s office, health maintenance organization, or a hospital outpatient department were categorized as having a usual source of care. Respondents who indicated that they had no usual source of care or obtained their typical health care through an emergency room were categorized as having no usual source of care. We grouped self-reported educational levels into four categories: less than high school, graduated high school, some college, and graduated college with or without professional or graduate school. We categorized respondents into those with insurance coverage, defined as individuals with private insurance, military insurance, other governmental insurance, Medicare or Medicaid, and those reporting no insurance coverage whatsoever.
Screening rates were stratified by family history and race. We only included respondents whose self-reported race was either “white” or “black.” We did not include respondents reporting other races within the analysis, as these groups were small. We used χ2 tests to compare screening rates between groups.
To determine the impact of race on early initiation of CRC screening in increased risk individuals, we constructed a separate logistic multivariate model for each CRC screening outcome (endoscopy within the preceding 10 years and FOBT within the past year). These models were adjusted for potential confounders to CRC screening including age, race, family history, gender, education, insurance status, and having a usual source of care.24 To further determine the impact of a positive family history for white and African-American respondents, we created two additional logistic multivariate models which were stratified by race. All data analyses were weighted to reflect national population estimates. To account for the NHIS multistage probability sample design, standard errors were adjusted using SAS-callable SUDAAN software, version 9 (Research Triangle Institute, Research Triangle Park, NC).
RESULTS
Overall, 279 respondents (5.4%: 95% C.I., 4.7, 6.2) reported having one or more first-degree relatives affected with CRC. African Americans and whites, aged 40 to 49 years, had similar rates of positive family histories for CRC (3.9%: 95% C.I., 2.5, 6.1 vs 5.6%: 95% C.I., 5.0, 6.4, P-value = .09). Among individuals with a positive family history, 67 (27.9%: 95% C.I., 22.1, 34.5) white respondents had undergone an endoscopic procedure within the previous 10 years compared to 3 (9.3%: 95% C.I., 1.7, 37.9) African-American respondents (P = .03). Among individuals without affected first-degree relatives, whites were more likely to have undergone an endoscopic procedure when compared to African Americans (10.5%: 95% C.I., 9.54, 11.6 vs 7.6%: 95% C.I., 5.8, 9.9, P-value = .01). Rates of FOBT screening for individuals with and without a family history of CRC are presented in Table 1.
Table 1.
Participation in Colorectal Cancer Screening Procedures in individuals Ages 40 to 49 Years Categorized by Family History
| Endoscopy within past 10 years | Fecal occult blood testing within past 1 year* | ||||||
|---|---|---|---|---|---|---|---|
| White | African American | P | White | African American | P | ||
| Positive family history | Percent, (95% CI) | 27.9% (22.1, 34.5) | 9.3% (1.7, 37.9) | .03 | 10.8% (7.0, 16.3) | 3.5% (0.74, 15.2) | .08 |
| N | 67/248 | 3/31 | 24/248 | 2/31 | |||
| No family history | Percent, (95% CI) | 10.5% (9.54, 11.6) | 7.6% (5.8, 9.9) | .01 | 6.0% (5.2, 6.9) | 4.6% (3.3, 6.4) | .13 |
| N | 462/4418 | 69/867 | 253/4387 | 43/863 | |||
*Denominator excludes 35 individuals (0.6%) with missing information on fecal occult blood testing.
Because endoscopic procedures can be preformed for diagnostic and screening reasons, endoscopy procedures were further categorized into screening procedures; defined as those where the respondent reported that the procedure had been performed as “part of a routine physical exam/screening test” or “family history” and diagnostic procedures, defined as “because of a specific problem,” “follow-up test of an earlier test/screening test,” or “other.” Overall, 49 endoscopies (70.5%: 95% C.I., 58.0, 80.5) performed in individuals with affected first-degree relatives were performed for screening purposes. White respondents with a positive family history reported 46 endoscopies (69.5%: 95% C.I., 56.9, 79.8) performed for screening purposes while African American respondents reported 3 endoscopic procedures (100%) performed for screening purposes (P = .26). In white respondents with positive family histories for CRC, 26 (41.1%: 95% C.I., 26.4, 53.9) reported family history as the reason for undergoing the endoscopy procedure compared to no African-American respondents (P = .25).
In the full model including all eligible subjects (n = 5,564), after adjusting for patient age, family history, gender, insurance status, education, and having a usual source of care, white respondents had an odds of 1.38 (95% C.I., 1.01, 1.87) of having undergone an endoscopic procedure within the past 10 years compared to African-Americans (P = .04) (Table 2). Individuals with a family history of CRC had an increased odds of 3.05 (95% C.I., 2.21, 4.20) of being current with endoscopy compared to individuals without family histories (P < .0001). In the adjusted models stratified by race, white individuals with an affected first degree relative with CRC had an odds of 3.21 (95% C.I., 2.31, 4.46) of being up-to-date with endoscopic screening when compared to white individuals with no affected relatives, while African-American respondents with a positive family history had an odds of 1.05 (95% C.I., 0.15, 7.21) of current with endoscopy compared to African Americans with no affected relatives. Results of the analysis using FOBT as the outcome are presented in Table 2. Family history was associated with an increased odds of being up to date with FOBT testing in white individuals but not in African-American individuals; however, in the full adjusted model, white individuals were not statistically more likely to have undergone FOBT than African Americans (OR = 1.23: 95% C.I., 0.83, 1.83).
Table 2.
Association of Colorectal Cancer Family History with Early-Initiation of Colorectal Cancer Screening interventions in Individuals Ages 40 to 49 Years*
| Characteristic | Endoscopy within past 10 years | |||
|---|---|---|---|---|
| Full model N = 5564 OR† (95% CI) | White N = 4,666 OR (95% CI) | African American N = 898 OR (95% CI) | ||
| Race | White | 1.38 (1.01, 1.87) | ||
| African American | 1.00 | |||
| Family history | Positive family history | 3.05 (2.21, 4.20) | 3.21 (2.31, 4.46) | 1.05 (0.15, 7.21) |
| No family history | 1.00 | 1.00 | 1.00 | |
| Gender | Female | 0.81 (0.66, 0.98) | 0.84 (0.69, 1.03) | 0.55 (0.31, 0.95) |
| Male | 1.00 | 1.00 | 1.00 | |
| Education level | College or prof grad | 1.53 (1.04, 2.26) | 1.62 (1.06, 2.48) | 0.98 (0.27, 3.55) |
| Some college | 1.42 (0.96, 2.10) | 1.45 (0.93, 2.24) | 1.55 (0.63, 3.83) | |
| High school grad | 1.09 (0.74, 1.60) | 1.17 (0.76, 1.81) | 0.61 (0.22, 1.65) | |
| <High school | 1.00 | 1.00 | 1.00 | |
| Insurance status | None | 0.81 (0.51, 1.27) | 0.80 (0.48, 1.32) | 0.96 (0.37, 2.47) |
| Any insurance | 1.00 | 1.00 | 1.00 | |
| Usual source of care | Yes | 2.85 (1.88, 4.31) | 2.70 (1.75, 4.17) | 5.50 (1.37, 22.12) |
| No | 1.00 | 1.00 | 1.00 | |
| FOBT‡ within past 1 year | ||||
| Race | White | 1.23 (0.83, 1.83) | ||
| African American | 1.00 | |||
| Family history | Positive family history | 1.62 (1.00, 2.64) | 1.71 (1.03, 2.84) | 0.76 (0.14, 3.99) |
| No family history | 1.00 | 1.00 | 1.00 | |
| Gender | Female | 1.23 (0.94, 1.60) | 1.21 (0.92, 1.60) | 1.37 (0.61, 3.06) |
| Male | 1.00 | 1.00 | 1.00 | |
| Education level | College or prof grad | 2.88 (1.55, 5.36) | 2.50 (1.28, 4.88) | 7.47 (1.93, 28.92) |
| Some college | 1.90 (1.01, 3.58) | 1.68 (0.84, 3.35) | 4.20 (1.04, 16.92) | |
| High school grad | 1.84 (0.97, 3.48) | 1.65 (0.83, 3.28) | 3.09 (0.75, 12.70) | |
| <High school | 1.00 | 1.00 | 1.00 | |
| Insurance status | None | 0.91 (0.52, 1.60) | 0.84 (0.44, 1.61) | 1.25 (0.35, 4.52) |
| Any insurance | 1.00 | 1.00 | 1.00 | |
| Usual source of care | Yes | 4.48 (2.32, 8.65) | 6.90 (2.97, 16.03) | 1.01 (0.29, 3.52) |
| No | 1.00 | 1.00 | 1.00 | |
*All models adjusted for age (continuous), family history, gender, educational level, insurance status, income, and having a usual source of care.
†OR = odds ratio.
‡Fecal occult blood testing.
When defining current with endoscopic procedures as having undergone at least one procedure within the past 5 years, having a positive family history was associated with an adjusted odds of 3.16 (95% C.I., 2.25, 4.45) when compared to an individual with no affected relatives for being current with endoscopy within the full model (data not shown). Race was not a significant predictor of endoscopy screening, with whites having an adjusted odds of 1.20 (95% C.I., 0.86, 1.67) of having undergone an endoscopic procedure within the preceding 5 years when compared to African Americans. In the models stratified by race, the adjusted odds ratio for being having undergone an endoscopic procedure within the previous 5 years in individuals with a family history of CRC when compared to individuals without a family history was 3.34 (95% C.I., 2.36, 4.74) and 1.20 (95% C.I., 0.18, 8.26) in whites and African Americans, respectively.
For respondents aged 40 to 49 years, self-perceived cancer risk was compared in individuals with affected first-degree relatives and those with no affected relatives (Fig. 2). In individuals with positive family histories of CRC, 201 white respondents (82.5%: 95% C.I., 76.9, 87.0) compared to 12 African-American respondents (36.5%: 95% C.I., 19.6, 57.6) perceived themselves as having a medium or high likelihood of developing cancer (P = .004). In individuals with no family history of CRC, 1,924 (46.6%: 95% C.I., 44.9, 48.4) whites and 276 (33.3%: 95% C.I., 29.4, 37.4) African Americans perceived themselves as at medium or high likelihood of developing cancer (P < .0001).
Figure 2.
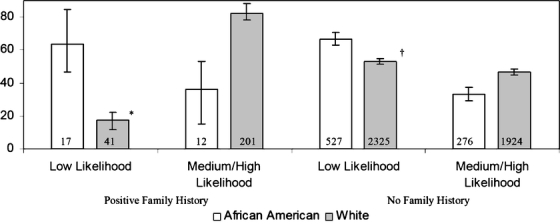
Respondent’s with positive family histories for colorectal cancer self-perceived likelihood of developing cancer compared to respondents with no family history. Numbers displayed within the bars are the sample size for each category *P-value = .004 †P-value < .0001.
CONCLUSION
We found that approximately 1 in 20 white and African-American individuals who were aged 40 to 49 were potential candidates for early initiation of CRC screening based on their family history. Screening rates were variable with African-American respondents being less likely to have undergone CRC screening compared to whites. After adjusting for age, gender, insurance, education, and having a usual provider, a positive family history was an important predictor of being up to date with a CRC screening procedure for whites but less so for African Americans. Finally, substantially fewer African Americans with positive family histories for CRC perceived themselves to be at increased risk for developing cancer compared to white respondents.
Although many studies have determined the rates of CRC screening in average risk individuals, only a few studies exist which have evaluated rates in individuals at increased risk. In one survey of provider practice patterns, 85% of gastroenterologists and 72% of primary care providers noted that they chose age 40 as the age to initiate screening for individuals with a family history of CRC.29 Despite these high self-reported rates of screening, our study found rates that were much lower. This is likely because of two factors. First, 15% of NHIS respondents reported having no usual source for heath care, an important factor in CRC screening.22 Second, although providers may follow appropriate screening recommendations when they have identified an individual at increased risk, family history information is collected in less than half of primary care visits 30, and when obtained, the information is often of poor quality and not useful for cancer risk assessment.31 Thus, it is possible that health care providers may not be identifying all individuals who are at increased risk because of their family histories.
We are unable to determine in our study whether family history information is less likely to be collected and acted upon from African-American patients compared to white patients or whether African-American patients are less likely to be motivated to seek screening based on their family history. Provider-specific factors, such as time constraints, competing patient demands, or knowledge deficits regarding clinical genetics could impact whether providers actively solicit family history information; however, limited evidence exists on this topic. Some studies have suggested that African-American women are less likely to be informed of the increased breast cancer risk associated with their family history; however, this finding has not been consistent.32,33 It is unknown if these differences also exist for CRC.
Patient-specific factors additionally contribute to how family history information affects medical care. It is clear that different cultures have different views on what constitutes a family unit and the appropriateness of sharing family history information with nonfamily members.34 In general, women with a positive family history of breast cancer undergo screening more frequently than women without a family history 35; however, this relationship has not been as reliable for African-American women.36,37 How knowledge of one’s family history impacts perceived cancer risk and subsequent preventive behaviors is not entirely understood.35,38,39 In our study, no African Americans noted family history as the reason for undergoing screening compared to 41% of white participants who described this as a factor. It is possible that African-American participants did not indicate family history as a reason for undergoing CRC screening because they may have perceived the risk associated with a positive family history as less important with regard to cancer risk when compared to white participants. In our study, fewer African Americans with affected relatives perceive themselves as at an increased risk for cancer compared to whites, although these results should be interpreted with caution as we did not exclude individuals with family histories for other cancer, such as breast or prostate, which may have a greater impact on perceived cancer risk than CRC.
There are several limitations to our study; most notable is that the NHIS Cancer Control Module only recorded information pertaining to first-degree relatives. This strategy was appropriate as previous evidence has indicated that self-reported family history information for colorectal, breast, and prostate cancer is highly accurate for first-degree relatives but becomes less so for second- and third-degree relatives.40 In addition, the NHIS does not inquire about a family history of adenomatous polyps. It is unclear how many individuals classified as having no family history were being screened as a result of affected second-degree relatives or a family history of adenomatous polyps. A second limitation of our study is the age of the data source. The most recent NHIS Cancer Control Module was administered in 2000. Several cancer-screening guidelines have recently changed, and we were only able to determine screening in compliance with 1997 guidelines. We also relied on patient self-report of CRC screening procedures as opposed to actual medical records to determine procedure rates. Several studies have suggested that patient self-report may overestimate colorectal screening rates and underestimate screening intervals.41–43 Our regression coefficients may also be biased by endogeneity, particularly if having a family history of CRC impacts an individuals desire to obtain health insurance or establish a usual source of care. Finally, although the NHIS includes data on over 30,000 individuals, the number of respondents, aged 40 to 49 with a positive family history, represented a relatively small subset of this population, and as such, our confidence intervals were large and our power somewhat limited to detect a true difference.
In conclusion, 1 in 20 individuals, aged 40 to 49 years, are candidates for early initiation of CRC screening. African-American individuals undergo early initiation of CRC screening at lower rates when compared to whites, with family history having little to no association with screening. Future work will be needed to determine how cultural differences impact risk perception and health-seeking behaviors in individuals with positive family histories and if family history of colorectal cancer may motivate individuals to undergo screening. Answers to these questions will be necessary if providers wish to gain maximal utility from family history information.
Acknowledgments
Acknowledgement The authors would like to acknowledge the Department of Veterans Affairs, Tennessee Valley Healthcare System, GRECC for its administrative support in the preparation of the manuscript.
Financial Support Dr. Murff is supported by a Career Development Award through the Vanderbilt-Ingram Cancer Center SPORE in GI Cancer Grant (CA 95103) and is a VA Clinical Research Scholar, VA Clinical Research Center of Excellence. The funding organization had no role in the design or conduct of the study, interpretation of the data, and preparation or approval of the manuscript.
Potential Financial Conflicts of Interest None disclosed.
References
- 1.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. Dec 30 1993;329(27):1977–81. [DOI] [PubMed]
- 2.Selby JV, Friedman GD, Quesenberry CP, Jr., Weiss NS. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. Mar 5 1992;326(10):653–7. [DOI] [PubMed]
- 3.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. May 13 1993;328(19):1365–71. [DOI] [PubMed]
- 4.Muller AD, Sonnenberg A. Protection by endoscopy against death from colorectal cancer. A case-control study among veterans. Arch Intern Med. Sep 11 1995;155(16):1741–48. [DOI] [PubMed]
- 5.Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. Nov 30 2000;343(22):1603–7. [DOI] [PubMed]
- 6.Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. Nov 30 1996;348(9040):1472–77. [DOI] [PubMed]
- 7.SEER cancer incidence public-use database, 1973–2001; Mortality Data 1969–2001. Bethesda, Maryland, U.S. Department of Health and Human Services. 2004.
- 8.Burt RW. Colon cancer screening. Gastroenterology. Sep 2000;119(3):837–53. [DOI] [PubMed]
- 9.Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale—update based on new evidence. Gastroenterology. Feb 2003;124(2):544–60. [DOI] [PubMed]
- 10.U.S. Preventive Services Task Force. Screening for colorectal cancer: recommendation and rationale. Ann Intern Med. Jul 16 2002;137(2):129–31. [DOI] [PubMed]
- 11.Smith RA, von Eschenbach AC, Wender R, et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. Also: update 2001—testing for early lung cancer detection. CA Cancer J Clin. 2001;51(1):38–75; quiz 77–80. [DOI] [PubMed]
- 12.Eisen GM, Weinberg DS. Narrative review: screening for colorectal cancer in patients with a first-degree relative with colonic neoplasia. Ann Intern Med. Aug 2 2005;143(3):190–8. [DOI] [PubMed]
- 13.Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. Oct 2001;96(10):2992–3003. [DOI] [PubMed]
- 14.Fuchs CS, Giovannucci EL, Colditz GA, Hunter DJ, Speizer FE, Willett WC. A prospective study of family history and the risk of colorectal cancer. N Engl J Med. 1994;331(25):1669–74. [DOI] [PubMed]
- 15.Polite BN, Dignam JJ, Olopade OI. Colorectal cancer and race: understanding the differences in outcomes between African Americans and whites. Med Clin North Am. Jul 2005;89(4):771–93. [DOI] [PubMed]
- 16.Wu XC, Andrews PA, Correa CN, et al. Breast cancer: incidence, mortality, and early detection in Louisiana, 1988–1997. J La State Med Soc. Apr 2001;153(4):198-209. [PubMed]
- 17.Clegg LX, Li FP, Hankey BF, Chu K, Edwards BK. Cancer survival among US whites and minorities: a SEER (Surveillance, Epidemiology, and End Results) Program population-based study. Arch Intern Med. Sep 23 2002;162(17):1985–93. [DOI] [PubMed]
- 18.Wee CC, McCarthy EP, Phillips RS. Factors associated with colon cancer screening: the role of patient factors and physician counseling. Prev Med. Jul 2005;41(1):23–9. [DOI] [PubMed]
- 19.O’Malley AS, Forrest CB, Feng S, Mandelblatt J. Disparities despite coverage: gaps in colorectal cancer screening among Medicare beneficiaries. Arch Intern Med. Oct 10 2005;165(18):2129–35. [DOI] [PubMed]
- 20.Cooper GS, Koroukian SM. Racial disparities in the use of and indications for colorectal procedures in Medicare beneficiaries. Cancer. Jan 15 2004;100(2):418–24. [DOI] [PubMed]
- 21.McMahon LF, Jr., Wolfe RA, Huang S, Tedeschi P, Manning W, Jr., Edlund MJ. Racial and gender variation in use of diagnostic colonic procedures in the Michigan Medicare population. Med Care. Jul 1999;37(7):712–7. [DOI] [PubMed]
- 22.Swan J, Breen N, Coates RJ, Rimer BK, Lee NC. Progress in cancer screening practices in the United States: results from the 2000 National Health Interview Survey. Cancer. Mar 15 2003;97(6):1528–40. [DOI] [PubMed]
- 23.Rao RS, Graubard BI, Breen N, Gastwirth JL. Understanding the factors underlying disparities in cancer screening rates using the Peters–Belson approach: results from the 1998 National Health Interview Survey. Med Care. Aug 2004;42(8):789–800. [DOI] [PubMed]
- 24.Hiatt RA, Klabunde C, Breen N, Swan J, Ballard-Barbash R. Cancer screening practices from National Health Interview Surveys: past, present, and future. J Natl Cancer Inst. Dec 18 2002;94(24):1837–46. [DOI] [PubMed]
- 25.2000 National Health Interview Survey (NHIS) Public Use Data Release: NHIS Survey Description. Division of Health Interview Statistics, National Center for Health Statistics, Hyattsville, MD. March 2002.
- 26.Byers T, Levin B, Rothenberger D, Dodd GD, Smith RA. American Cancer Society guidelines for screening and surveillance for early detection of colorectal polyps and cancer: update 1997. American Cancer Society Detection and Treatment Advisory Group on Colorectal Cancer. CA Cancer J Clin. May–Jun 1997;47(3):154–60. [DOI] [PubMed]
- 27.Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology. Feb 1997;112(2):594–642. [DOI] [PubMed]
- 28.Guide to Clinical Preventive Services, Report of the U.S. Preventive Services Task Force. 2nd Edition ed. Alexandria, Virginia: International Medical Publishing, Inc.; 1996.
- 29.Schroy PC, 3rd, Barrison AF, Ling BS, Wilson S, Geller AC. Family history and colorectal cancer screening: a survey of physician knowledge and practice patterns. Am J Gastroenterol. Apr 2002;97(4):1031–6. [DOI] [PubMed]
- 30.Acheson L, Wiesner G, Zyzanski S, Goodwin M, Strange K. Family history-taking in community family practice: implications for genetic screening. Genet Med. May/June 2000;2(3):180–5. [DOI] [PubMed]
- 31.Murff HJ, Byrne D, Syngal S. Cancer risk assessment quality and impact of the family history interview. Am J Prev Med. Oct 2004;27(3):239–45. [DOI] [PubMed]
- 32.Audrain J, Lerman C, Rimer B, Cella D, Steffens R, Gomez-Caminero A. Awareness of heightened breast cancer risk among first-degree relatives of recently diagnosed breast cancer patients. The High Risk Breast Cancer Consortium. Cancer Epidemiol Biomarkers Prev. Jul–Aug 1995;4(5):561–5. [PubMed]
- 33.Royak-Schaler R, Klabunde CN, Greene WF, et al. Communicating breast cancer risk: patient perceptions of provider discussions. Medscape Womens Health. Mar–Apr 2002;7(2):2. [PubMed]
- 34.Meiser B, Eisenbruch M, Barlow-Stewart K, Tucker K, Steel Z, Goldstein D. Cultural aspects of cancer genetics: setting a research agenda. J Med Genet. Jul 2001;38(7):425–9. [DOI] [PMC free article] [PubMed]
- 35.McCaul KD, Branstetter AD, Schroeder DM, Glasgow RE. What is the relationship between breast cancer risk and mammography screening? A meta-analytic review. Health Psychol. Nov 1996;15(6):423–29. [DOI] [PubMed]
- 36.Husaini BA, Sherkat DE, Bragg R, et al. Predictors of breast cancer screening in a panel study of African American women. Women Health. 2001;34(3):35–51. [DOI] [PubMed]
- 37.West DS, Greene PG, Kratt PP, et al. The impact of a family history of breast cancer on screening practices and attitudes in low-income, rural, African American women. J Womens Health (Larchmt). Oct 2003;12(8):779–87. [DOI] [PubMed]
- 38.Katapodi MC, Lee KA, Facione NC, Dodd MJ. Predictors of perceived breast cancer risk and the relation between perceived risk and breast cancer screening: a meta-analytic review. Prev Med. Apr 2004;38(4):388–402. [DOI] [PubMed]
- 39.Smith BL, Gadd MA, Lawler C, et al. Perception of breast cancer risk among women in breast center and primary care settings: correlation with age and family history of breast cancer. Surgery. Aug 1996;120(2):297–303. [DOI] [PubMed]
- 40.Murff HJ, Spigel DR, Syngal S. Does this patient have a family history of cancer? An evidence-based analysis of the accuracy of family cancer history. JAMA. Sep 22 2004;292(12):1480–9. [DOI] [PubMed]
- 41.Gordon NP, Hiatt RA, Lampert DI. Concordance of self-reported data and medical record audit for six cancer screening procedures. J Natl Cancer Inst. Apr 7 1993;85(7):566–70. [DOI] [PubMed]
- 42.Lipkus IM, Rimer BK, Lyna PR, Pradhan AA, Conaway M, Woods-Powell CT. Colorectal screening patterns and perceptions of risk among African-American users of a community health center. J Commun Health. Dec 1996;21(6):409–27. [DOI] [PubMed]
- 43.Hiatt RA, Perez-Stable EJ, Quesenberry C, Jr., Sabogal F, Otero-Sabogal R, McPhee SJ. Agreement between self-reported early cancer detection practices and medical audits among Hispanic and non-Hispanic white health plan members in northern California. Prev Med. May 1995;24(3):278–85. [DOI] [PubMed]


