Abstract
Background
Aspirin reduces mortality for men and women with coronary heart disease (CHD). Previous research suggests women with acute coronary syndromes receive less aggressive care, including less frequent early administration of aspirin. The presence of gender differences in aspirin use for secondary prevention is less clear.
Objective
To determine if a gender difference exists in the use of aspirin for secondary prevention among individuals with CHD.
Design
We analyzed data from the nationally representative 2000–2002 Medical Expenditure Panel Surveys to determine the prevalence of regular aspirin use among men and women with CHD.
Participants
Participants, 1,869, 40 years and older who reported CHD or prior myocardial infarction.
Results
Women were less likely than men to use aspirin regularly (62.4% vs 75.6%, p < .001) even after adjusting for demographic, socioeconomic and clinical characteristics (adjusted OR = 0.62, 95% CI, 0.48–0.79). This difference narrowed but remained significant when the analysis was limited to those without self-reported contraindications to aspirin (79.8% vs 86.4%, P = .002, adjusted OR = 0.68, 95% CI, 0.48–0.97). Women were more likely than men to report contraindications (20.5% vs 12.5%, P < .001). Differences in aspirin use were greater between women and men with private health insurance (61.8% vs 79.0%, P < .001, adjusted OR = 0.48, 95% CI, 0.35–0.67) than among those with public coverage (62.5% vs 70.7%, P = .04, adjusted OR = 0.74, 95% CI, 0.50–1.11) (P < .001 for gender–insurance interaction).
Conclusion
We found a gender difference in aspirin use among patients with CHD not fully explained by differences in patient characteristics or reported contraindications. These findings suggest a need for improved secondary prevention of cardiovascular events for women with CHD.
Key words: coronary heart disease, myocardial infarction, aspirin, secondary prevention, gender, insurance, health, medical expenditure panel survey
Introduction
Aspirin confers protection against myocardial infarction (MI), stroke, and other vascular events and reduces mortality for men and women with coronary heart disease (CHD).1 Although the utility of aspirin for the primary prevention of vascular events in otherwise healthy women is unclear,2–4 strong evidence supports the benefits of aspirin for women with CHD.1,5 American College of Cardiology (ACC)/American Heart Association (AHA) guidelines therefore recommend daily aspirin for all patients with atherosclerotic cardiovascular disease unless contraindicated.5,6
Gender differences in the management of acute coronary syndromes, including the use of thrombolytics, angioplasty, beta-blockers, and aspirin, are well documented.7–13 Given aspirin’s clear benefits for both women and men with CHD, gender differences in its use that persist beyond the inpatient experience could additionally impact the health of women with CHD on a population level. However, less is known about gender differences in outpatient care of CHD in general,14–17 and few studies have specifically assessed gender differences in aspirin use among men and women with CHD.18,19
Estimating aspirin use poses a special challenge. Aspirin’s low cost, over-the-counter availability, and widely known benefits for patients with CHD distinguish it as a unique medication whose patterns of use are difficult to measure and may be less subject to mechanisms proposed to explain gender disparities in other medical treatments.11,16,20 For example, differences in prescription drug coverage are less likely to affect aspirin use. Consequently, gender differences in aspirin use may not correspond to those demonstrated for other aspects of care for CHD.
Most studies addressing gender differences in the outpatient care of patients with CHD were limited to local populations or particular health systems14,15,17,21,22 and others lacked outpatient measures of aspirin use.7,12,21 Two studies utilized national data but relied on ambulatory visit documentation that may not accurately measure aspirin use, and these were estimated per visit reporting rates rather than population prevalences.18,19 As a result, estimates of aspirin use vary widely, from 33%19 to 83%17 to 97%,15 and the significance of gender differences in the general population of patients with CHD remains unclear.
Therefore, to more clearly define population-level differences in aspirin use between women and men with CHD and to investigate patient characteristics that may contribute to or modify such differences, we used data from a nationally representative survey to compare self-reported aspirin use by gender in adults with CHD.
Subjects and Methods
Data Source
We analyzed data from the 2000, 2001, and 2002 Medical Expenditure Panel Survey (MEPS), a survey sponsored by the Agency for Healthcare Research and Quality and the National Center for Health Statistics. MEPS is designed to provide nationally representative estimates of health care use, expenditures, sources of payment, and insurance coverage.23,24 Starting in 2000, condition-specific questions were included to assess whether care is consistent with practice guidelines.25 Each panel of respondents is drawn from the previous year’s National Health Interview Survey, and respondents are repeatedly interviewed in person 5 times over a 2 1/2-year period using computer-assisted personal interviewing technology. Response rates for the three survey years varied between 64.7% and 66.3%.23 The MEPS survey design is complex, involving multistage sampling and producing stratified and clustered data. Information is provided to account for the complex survey design, including weights to adjust for nonresponse and disproportionate sampling. Each year of data provides a representative sample of the U.S. civilian noninstitutionalized population. MEPS has been used previously to study various issues relating to women’s health, gender disparities, and cardiovascular disease.26–28 Because this study used publicly available anonymous data, the Institutional Review Board of Brigham and Women’s Hospital deemed it exempt from review.
Study Population
Our study cohort included participants 40 years of age or older at the time of the survey who reported a diagnosis of coronary heart disease (CHD) or a previous heart attack or myocardial infarction (MI). Individuals with missing values for demographic or clinical variables were excluded.
Study Variables
Aspirin use and covariates were ascertained from self-reports. Participants or their proxies were asked two questions to determine the presence of CHD: “Have you ever been told by a doctor or other health professional that you had [coronary heart disease],” or “[a heart attack, also called myocardial infarction or MI]?” Those who replied yes to either of these questions were asked, “Do you take aspirin everyday or every other day?” Respondents with MI or CHD who did not report regular aspirin use were asked, “Do you have a health problem or condition that makes taking aspirin unsafe?” Those who reported such a contraindication were specifically asked “Is that problem stomach-related or something else?” Neither the dose nor the duration of aspirin use was determined, and specific contraindications were not characterized beyond whether they were “stomach-related.”
Demographic and socioeconomic covariates included age (at the time of the interview), gender, health insurance status (private insurance, public with no private insurance, or uninsured), education (<high school education, high school graduate, or >high school education), poverty category (income 0–124%, 125–199%, 200–399%, or 400+% of local poverty level adjusted for family size), and census region (South, Midwest, West, and Northeast). Race and ethnicity were categorized as non-Hispanic white, non-Hispanic black, Hispanic, or other, using responses to two questions about race (white, black, or other) and ethnicity (Hispanic or non-Hispanic).
Presence of other medical problems including asthma, diabetes, and hypertension, was also determined by self-report of diagnoses. Finally, measures of preventive counseling and regular healthcare access were adapted from three questions: “Has a doctor or other health professional ever advised you to...[exercise more]”; “...[eat fewer high fat or high cholesterol foods]”; and “About how long has it been since your blood pressure was checked by a doctor, nurse or other health professional?” A dichotomous variable (blood pressure checked within the last year) was created from the answer to the last of these questions. Year of participation (2000, 2001, or 2002) did not significantly predict aspirin use (P = .50) and is not included in the analysis.
Statistical Analysis
All demographic, socioeconomic, and clinical characteristics were compared by gender using X2 tests for categorical variables.
Logistic regression was used to estimate unadjusted and adjusted odds ratios for aspirin use. Because effect estimates were not appreciably altered by the inclusion of the 3 indicators of preventive counseling and health care access, these variables were removed from the final multiple logistic regression model. This allowed 9 participants who lacked data for these three variables to be included in the final adjusted analyses. Age was included as both a continuous and categorical (<65, 65–74, and >74 years old) variable. As results were similar, only results based on age as a categorical variable are presented. Because MEPS lacks other measures of clinical appropriateness of aspirin use to validate self-reported contraindications, we conducted adjusted analyses for both the full cohort and the subgroup excluding those reporting a contraindication to aspirin.
All two-way interactions with gender were analyzed. A statistically significant two-way interaction was found only between type of insurance coverage and the effect of gender on aspirin use (P < .001). The analysis was stratified accordingly by insurance coverage. Because previous studies have suggested that younger women may be especially likely to receive less intensive care after an MI,21 and because Medicare eligibility modifies the distinction between private and public insurance for adults 65 or older, the adjusted analysis was further stratified by a dichotomous age variable (<65 and ≥65 years old). A three-way interaction assessed between gender, insurance status, and this age indicator was significant (P < .05). Only 3 participants, 65 years or older, were uninsured, so results from this group are not reported.
All analyses were conducted with SAS 9.01 (SAS Institute, Cary, NC) and SUDAAN release 8.0 (Research Triangle Institute, Research Triangle Park, NC) statistical software to account for the complex survey design.
Results
The 2000–2002 MEPS included 25,486 participants, 40 years of age or older, 1,938 (7.6%) of whom reported either a previous MI (1303, 5.1%) or CHD alone (635, 2.5%). Of those, 69 (3.6%) were excluded from the analysis because of missing data. There were no statistically significant observed differences between those included and those excluded because of missing data (all P > .10). Overall, 1,869 participants were included in the analysis.
Findings from descriptive comparisons by gender are summarized by Table 1. The prevalence of regular aspirin use was 70.5% in the study cohort, and 84.0% among those without a contraindication to taking aspirin. In both unadjusted and adjusted comparisons, women were significantly less likely than men to take aspirin (62.4% vs 75.6%, P < .001, adjusted OR = 0.62, 95% CI, 0.48–0.79) as shown in Table 2. This difference persisted when the analysis was limited to the 84.4% of participants without reported contraindications to aspirin (79.8% vs 86.4%, p = .002, adjusted OR = 0.68, 95% CI, 0.48–0.97), but the absolute unadjusted gender difference narrowed from 13.2% to 6.6%. Among those without reported contraindications, both older and younger participants were significantly less likely to take aspirin than adults age 65–74; Hispanic adults were less likely than non-Hispanic whites to be regular aspirin users; and participants who reported a previous myocardial infarction or hypertension were more likely to take aspirin regularly. Also notable was a strong stepwise relationship between higher income and greater aspirin use.
Table 1.
Descriptive Statistics by Gendera
| Men | Percentage (%) | Women | Percentage (%) | P valueb | |
|---|---|---|---|---|---|
| N | N | ||||
| Total | 1,098 | 771 | |||
| Age, years | |||||
| <65 | 464 | 43.0 | 253 | 30.0 | <0.001 |
| 65–74 | 335 | 29.8 | 213 | 28.6 | |
| >75 | 299 | 27.3 | 305 | 41.4 | |
| Race/ethnicity | |||||
| Non-Hispanic White | 849 | 84.9 | 529 | 79.6 | <0.001 |
| Non-Hispanic Black | 114 | 6.9 | 150 | 13.0 | |
| Hispanic | 105 | 5.2 | 71 | 4.2 | |
| Other | 30 | 3.1 | 21 | 3.2 | |
| Insurance | |||||
| Private | 686 | 65.6 | 373 | 54.0 | <0.001 |
| Public | 361 | 30.5 | 362 | 42.4 | |
| Uninsured | 51 | 3.9 | 36 | 3.6 | |
| Percent of federal poverty level | |||||
| >400% | 398 | 38.7 | 145 | 21.8 | <0.001 |
| 200–399% | 333 | 31.5 | 181 | 25.5 | |
| 125–199% | 166 | 15.4 | 169 | 23.0 | |
| 0–124% | 201 | 14.5 | 276 | 29.7 | |
| Education level | |||||
| <HS education | 359 | 27.9 | 362 | 42.9 | <0.001 |
| High school graduate | 325 | 32.2 | 255 | 34.7 | |
| >HS education | 414 | 40.0 | 154 | 22.4 | |
| Census region | |||||
| Northeast | 174 | 17.4 | 127 | 18.9 | 0.17 |
| Midwest | 254 | 22.5 | 190 | 26.0 | |
| South | 475 | 42.2 | 334 | 39.3 | |
| West | 195 | 17.9 | 120 | 15.7 | |
| Diabetes mellitus | 301 | 25.8 | 272 | 32.4 | 0.01 |
| Asthma | 111 | 9.5 | 161 | 19.3 | <0.001 |
| Prior Myocardial Infarction | 759 | 69.5 | 498 | 64.9 | 0.04 |
| Hypertension | 768 | 69.5 | 612 | 77.6 | 0.001 |
| Advised to exercise morec | 807 | 72.7 | 509 | 65.7 | 0.003 |
| Advised to restrict high fat foodsc | 852 | 76.7 | 554 | 71.4 | 0.05 |
| Blood pressure checked in past yearc | 1,060 | 96.5 | 762 | 98.8 | 0.01 |
aUnweighted sample sizes are presented, but percentages were calculated using provided analytic weights. Because of rounding, percentages may not total 100.
bSignificance tests were performed with a X2 test and were adjusted for survey design.
cNine participants were excluded from analysis of at least one of the three variables reflecting health care access because of missing data.
Table 2.
Regular Aspirin Use by Socioeconomic and Clinical Characteristics
| Characteristics | All Participants | All Participants | Participants without contraindication to aspirin | |
|---|---|---|---|---|
| Unadjusted | Adjusteda | Adjusted | ||
| Percentage (%) | OR (95% CI)b | OR (95% CI) | ||
| Gender | Women | 62.4 | 0.62 (0.48–0.79) | 0.68 (0.48–0.97) |
| Men | 75.6 | 1.00 | 1.00 | |
| Age, years | <65 | 71.4 | 0.91 (0.67–1.24) | 0.57 (0.37–0.86) |
| 65–74 | 74.3 | 1.00 | 1.00 | |
| >75 | 65.9 | 0.74 (0.54–1.01) | 0.58 (0.38–0.88) | |
| Race/ethnicity | Non-Hispanic Black | 67.3 | 0.82 (0.58–1.18) | 0.66 (0.41–1.07) |
| Hispanic | 63.8 | 0.85 (0.55–1.32) | 0.56 (0.33–0.95) | |
| Other | 56.1 | 0.52 (0.29–0.92) | 0.30 (0.15–0.58) | |
| Non-Hispanic White | 71.9 | 1.00 | 1.00 | |
| Insurance | Uninsured | 61.3 | 0.72 (0.41–1.28) | 0.55 (0.29–1.05) |
| Public | 66.8 | 0.97 (0.73–1.28) | 0.96 (0.64–1.42) | |
| Private | 73.1 | 1.00 | 1.00 | |
| Percent of federal poverty level | >400% | 76.5 | 1.80 (1.24–2.62) | 2.85 (1.68–4.86) |
| 200%-399% | 74.4 | 1.70 (1.19–2.43) | 1.94 (1.21–3.12) | |
| 125%-199% | 66.2 | 1.31 (0.91–1.88) | 1.60 (0.98–2.61) | |
| 0-124% | 59.0 | 1.00 | 1.00 | |
| Education level | <High school education | 67.4 | 0.93 (0.67–1.29) | 1.04 (0.67–1.62) |
| High school graduate | 68.9 | 0.82 (0.60–1.12) | 0.82 (0.55–1.23) | |
| >High school education | 75.1 | 1.00 | 1.00 | |
| Census region | Northeast | 73.9 | 1.40 (0.91–2.15) | 1.41 (0.81–2.43) |
| Midwest | 71.5 | 1.24 (0.83–1.85) | 1.27 (0.76–2.10) | |
| South | 69.5 | 1.08 (0.74–1.58) | 1.41 (0.88–2.26) | |
| West | 67.6 | 1.00 | 1.00 | |
| Diabetes mellitus | Yes | 72.1 | 1.15 (0.87–1.52) | 1.45 (0.97–2.16) |
| No | 69.8 | 1.00 | 1.00 | |
| Asthma | Yes | 61.2 | 0.72 (0.52–1.00) | 0.78 (0.52–1.18) |
| No | 71.9 | 1.00 | 1.00 | |
| Prior Myocardial Infarction | Yes | 71.3 | 1.16 (0.91–1.50) | 1.38 (1.00–1.91) |
| No | 68.6 | 1.00 | 1.00 | |
| Hypertension | Yes | 72.1 | 1.50 (1.16–1.95) | 2.08 (1.48–2.93) |
| No | 66.1 | 1.00 | 1.00 | |
aThe adjusted multivariate model included gender, insurance status, percent of federal poverty level, education level, race/ethnicity, age, diabetes mellitus, asthma, prior myocardial infarction, hypertension, and census region. All results have been adjusted for the complex design of the survey and analytic weights.
bOR = odds ratio, CI = confidence interval.
Participants who reported that a health care provider had advised them to eat a healthier diet were more likely to take aspirin (75.0% vs 59.9%, P < .001; adjusted OR = 1.42, 95% CI, 1.06–1.90), as were those counseled to exercise more (74.9% vs 58.0% P < .001; adjusted OR = 1.72, 95% CI, 1.27–2.33), or whose health care provider had checked their blood pressure in the past year (70.9% vs 54.6%, P = .04; adjusted OR = 2.03, 95% CI, 0.99–4.14). However, including these variables in the regression model did not appreciably alter estimates, and there were no significant two-way interactions involving these covariates.
Differences in aspirin use between women and men were greatest among those with private health insurance (p < .001 for a gender-insurance status interaction) (Table 3). Among those with private insurance, women reported aspirin use less frequently than men. In contrast, women and men with public insurance reported aspirin use with similar frequency after adjusting for confounding characteristics. Compared to a similar prevalence of aspirin use among women with private (62.5%) and public coverage (61.8%), men with private coverage reported regular aspirin use with greater frequency (79.0%) than men with public insurance (70.7%). Results for the uninsured were difficult to interpret because of small sample size (n = 87), and therefore, are not reported. Inclusion of the three variables reflecting regular preventive care did not diminish this variation in gender differences by insurance status.
Table 3.
Regular Aspirin Use by Gender, Stratified by Insurance Status
| N | Unadjusted Percentage (%) | Unadjusted OR (95% CI)a | Adjusted ORb (95% CI) | |
|---|---|---|---|---|
| All participants | ||||
| Women | 771 | 62.4 | 0.54 (0.42–0.68) | 0.62 (0.48–0.79) |
| Men | 1,098 | 75.6 | ||
| Private insurance | ||||
| Women | 361 | 61.8 | 0.43 (0.32–0.59) | 0.48 (0.35–0.67) |
| Men | 686 | 79.0 | ||
| Public insurance | ||||
| Women | 362 | 62.5 | 0.69 (0.48–1.00) | 0.74 (0.50–1.11) |
| Men | 361 | 70.7 | ||
Prevalence of aspirin use for men and women, by insurance status, in the complete study cohort. Univariate and multivariate odds ratios (±95% confidence intervals) for aspirin use are presented, with men as the referent group. Results for uninsured participants are not presented because of the small sample size (n = 87).
aOR = odds ratio, CI = confidence interval.
bThe adjusted multivariate model included percent of federal poverty level, education level, race/ethnicity, age, diabetes mellitus, asthma, prior myocardial infarction, hypertension, and census region. All results have been adjusted for the complex design of the survey and analytic weights.
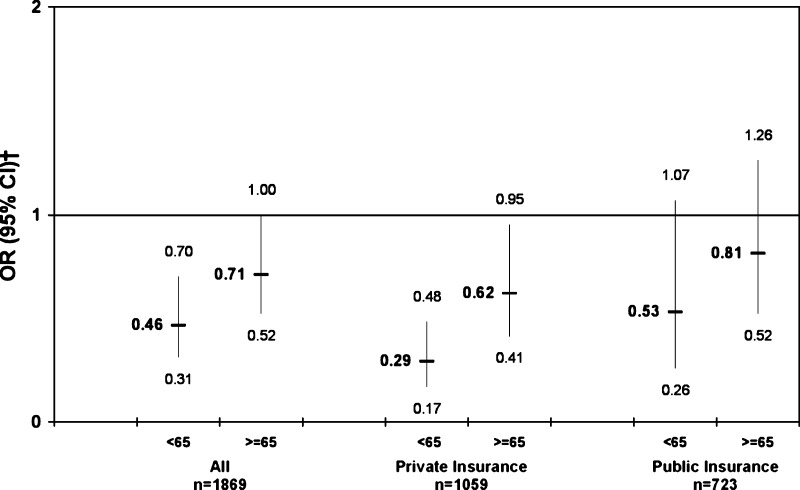
As shown in Figure 1, gender differences in aspirin use were greater among younger adults (P = .04 for gender–age interaction) when age was dichotomized as younger than 65 versus 65 or older. The modifying effect of insurance status on gender differences in aspirin use significantly differed by age (P = .047 for three-way gender–insurance–age interaction). The difference in aspirin use reported by privately insured men and women was more pronounced for those under 65 (59.3% vs 82.5%, P < .001, adjusted OR = 0.29, 95% CI, 0.17–0.48), than for older participants (63.0% vs 76.2%, P = .002, adjusted OR = 0.62, 95% CI, 0.41–0.95). In contrast, gender differences in aspirin use among publicly insured participants were not significant for either age group.
Figure 1.
Gender differences in regular aspirin use stratified by age and health insurance status, adjusted model*. Odds ratios for aspirin use in the complete cohort by gender, stratified by insurance status (men are the referent group). Results for uninsured participants are not presented because of small sample sizes (n = 84 and n = 3 for those <65 and ≥65 years old, respectively). *The adjusted multivariate model included gender, percent of federal poverty level, education level, race/ethnicity, diabetes mellitus, asthma, prior myocardial infarction, hypertension, and census region. Results have been adjusted for the complex design of the survey and analytic weights. †OR = odds ratio, CI = confidence interval.
Overall, 15.6% of the participants reported a contraindication to aspirin use. Those with asthma reported a greater prevalence of aspirin contraindications (21.6% vs 14.7%, P = .02). Older participants tended to be more likely to report a contraindication, although this finding was not statistically significant (12.6% for <65, 16.6% for 65–74, 18.2% for >74 years old, P = .08). Women were more likely than men to report a contraindication (20.5% vs 12.5%, P < .001). This was not attributable to the age difference between genders. Both women under 65 years old (19.4% vs 9.6%, P = .005) and those 65 years or older (21.0% vs 14.7%, P = .01) reported medical contraindications more often than men with CHD. The higher prevalence of asthma among women did not explain the difference, either. While aspirin contraindications were equally common for women and men with asthma (22.5% vs 20.5%, P = .77), women had a higher prevalence of contraindications (20.1% vs 11.6%, P < .001) among those without asthma. No other covariates were significantly associated with aspirin contraindications.
Approximately half (52.8%) of participants not taking aspirin reported a medical contraindication to its use. This figure was similar for women and men (54.6% vs 51.0%, P = .45) and both genders were equally likely to attribute a reported contraindication to ‘stomach problems’ (33.2% vs 28.1%, P = .38).
Discussion
In this nationally representative study, we found that women with CHD were significantly less likely than men to take aspirin regularly despite its clear indication. Our findings suggest that gender differences previously demonstrated in the treatment of acute coronary syndromes are also present in the chronic management of CHD and are significant at a population level. Extrapolation from findings of previous studies to the United States population is limited because of the specific populations studied and the methods used to assess aspirin use. Our study, therefore, offers more robust national estimates than studies that were limited to prescription data, hospital or ambulatory care of patients, localized geographic regions, or particular health systems.7,12,14,15,17–19,21,22
The gender difference in aspirin use remained significant after adjustment for numerous patient characteristics and self-reported contraindications. Our findings are, therefore, suggestive of an underlying disparity in the quality of care received by men and women with CHD. Underuse of aspirin would put women with CHD at an increased risk of preventable cardiovascular events and death. In conjunction with dissimilar acute treatment and possible sex-related biological differences, this gender difference in aspirin use for secondary prevention may help to explain why younger women have poorer outcomes than men in the first few years after an MI.21,29–32
While characterization of factors contributing to this potential disparity remains an area for future research, we did find that gender differences in aspirin use were most pronounced among younger men and women with private health insurance coverage and that women were more likely to report contraindications to aspirin use.
The high rate of aspirin use among non-elderly privately insured men may be attributable to unmeasured characteristics of working-age men, to gender differences in insurance benefits, or to features of private health plans such as mandatory reporting on standardized performance measures that may augment secondary prevention in the ambulatory care of men with CHD. Alternatively, men are more likely to receive ambulatory specialty cardiology care after an MI,33 and private plans may facilitate such referrals with greater ease and frequency.
A higher prevalence, real or perceived, of adverse effects or contraindications to aspirin among women could also explain our findings.35,36 Unexpectedly, women were more likely than men to report contraindications to aspirin use, and limiting the analysis to those without a contraindication to aspirin use narrowed the absolute gender gap. Gender was a stronger predictor of reported contraindications than age. This higher prevalence of contraindications to aspirin among women is puzzling. There is no clear gender difference in the prevalence of peptic ulcer disease or in mortality secondary to its complications.37,38 Aspirin use increases the risk of bleeding similarly for both men and women.3,4,39,40 Women were more likely to report asthma, which can constitute a contraindication to aspirin use,41,42 but even women without asthma were more likely to report a contraindication than their male counterparts. Other adverse effects such as easy bruising, hematuria, and epistaxis rarely prevent aspirin use.43,44
Gender differences in aspirin use could also be explained by uncertainty and related biases among treating physicians. For example, providers may generalize the unclear efficacy of aspirin in primary prevention for women at low risk of vascular events to women with known CHD.2–4,34
This study has several limitations. The low response rate for this survey (65%) may be attributable to the long duration of the survey or to the restriction of participants to the previous year’s National Health Interview Survey (NHIS) sample (there is only a 75–80% initial response of these participants to the MEPS survey). Our findings could represent a differential response by gender or aspirin use. Sample weights are designed to account for, but cannot guarantee the absence of, nonresponse bias.
Data were ascertained by self-report and our results could potentially be explained by reporting biases, such as over-reporting of CHD among women or aspirin use among men. While some data suggest that women may be less accurate in reporting a history of MI or cardiac disease,45 other studies have found no such difference or have reported higher accuracy among women.46,47 No significant gender differences have been demonstrated in studies of the accuracy of self-reports of other chronic diseases.48–51
Although the breadth of data in MEPS allowed for adjustment for many patient characteristics, we were unable to independently assess the clinical appropriateness of aspirin use, medication adherence, or patient preferences as potential explanations for our findings. Specifically, data on contraindications to aspirin use were limited, and the validity of self-reported contraindications could not be confirmed. As a result, we could not distinguish real contraindications indicative of appropriate nonuse from perceived contraindications suggestive of a need for greater patient education.
The contribution of non-adherence to recommended treatment to the observed gender difference could not be studied with the available data. Several recent papers have found that women with CHD are less likely to continue evidence-based therapies prescribed at discharge.17,52 However, the reasons for lower persistence were not investigated in these studies, and poorer adherence is only one potential explanation. Furthermore, many studies of adherence to prescribed treatments, generally, have demonstrated that gender has no relationship to adherence.53–55
In addition, because of the observational cross-sectional study design, we could not adjust for unobserved differences between men and women with CHD. There were significant gender differences in many of our measured covariates, and unmeasured variables could confound or explain our findings. Potential unmeasured confounders include the use of other antiplatelet agents or anticoagulants and health behaviors such as tobacco use. In addition, we were unable to assess differences in access and quality of care beyond the receipt of basic counseling and routine blood pressure monitoring. Concomitant differences observed in the receipt of specialty referrals or other recommended elements of care for CHD, for example, would bolster an argument that our findings reflect a true gender disparity. Therefore, while our findings are consistent with the provision of inferior care to women with CHD in the United States and suggest possible explanations for this deficit, further research is needed to address the limitations of this study and to elucidate modifiable factors of care to guide interventions.
Our findings demonstrate a difference in aspirin use between women and men with CHD, contribute to growing evidence of inferior care for women with CHD, suggest an additional explanation for poorer observed outcomes among women, and have important policy implications. Women are not receiving the full benefits of secondary prevention, and as a result, may be at greater risk for cardiovascular events and premature death. Thus, distinct benefits to the health of women with CHD may be realized through greater use of a very low-cost medication. Although further investigation is needed to better define the underlying causes of lower aspirin use among women, its remedy may require a multifaceted approach including patient and physician education, enhanced measurement and quality improvement initiatives by providers, health plans, and public insurance programs, and focused interventions at the point of care.
Acknowledgement
This work was supported (J.M.M.) by a fellowship from the Agency for Healthcare Research and Quality, Department of Health and Human Services (T32 HS00020-20).
Potential Financial Conflicts of Interest
None disclosed.
References
- 1.Collaborative overview of randomised trials of antiplatelet therapy-I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ 1994;308(6921):81–06. [PMC free article] [PubMed]
- 2.Aspirin for the primary prevention of cardiovascular events: recommendation and rationale. Ann Intern Med 2002;136(2):157–60. [DOI] [PubMed]
- 3.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352(13):1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 4.Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA. 2006;295(3):306–313. doi: 10.1001/jama.295.3.306. [DOI] [PubMed] [Google Scholar]
- 5.Mosca L, Appel LJ, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women. Circulation. 2004;109(5):672–693. doi: 10.1161/01.CIR.0000114834.85476.81. [DOI] [PubMed] [Google Scholar]
- 6.Smith SC, Jr, Blair SN, Bonow RO, et al. AHA/ACC Guidelines for Preventing Heart Attack and Death in Patients With Atherosclerotic Cardiovascular Disease: 2001 update. A statement for healthcare professionals from the American Heart Association and the American College of Cardiology. J Am Coll Cardiol. 2001;38(5):1581–1583. doi: 10.1016/S0735-1097(01)01682-5. [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin TJ, Soumerai SB, Willison DJ, et al. Adherence to national guidelines for drug treatment of suspected acute myocardial infarction: evidence for undertreatment in women and the elderly. Arch Intern Med. 1996;156(7):799–805. doi: 10.1001/archinte.156.7.799. [DOI] [PubMed] [Google Scholar]
- 8.Ayanian JZ, Epstein AM. Differences in the use of procedures between women and men hospitalized for coronary heart disease. N Engl J Med. 1991;325(4):221–225. doi: 10.1056/NEJM199107253250401. [DOI] [PubMed] [Google Scholar]
- 9.Barakat K, Wilkinson P, Suliman A, Ranjadayalan K, Timmis A. Acute myocardial infarction in women: contribution of treatment variables to adverse outcome. Am Heart J. 2000;140(5):740–746. doi: 10.1067/mhj.2000.110089. [DOI] [PubMed] [Google Scholar]
- 10.Yarzebski J, Col N, Pagley P, Savageau J, Gore J, Goldberg R. Gender differences and factors associated with the receipt of thrombolytic therapy in patients with acute myocardial infarction: a community-wide perspective. Am Heart J. 1996;131(1):43–50. doi: 10.1016/S0002-8703(96)90049-6. [DOI] [PubMed] [Google Scholar]
- 11.Rathore SS, Berger AK, Weinfurt KP, et al. Race, sex, poverty, and the medical treatment of acute myocardial infarction in the elderly. Circulation. 2000;102(6):642–648. doi: 10.1161/01.cir.102.6.642. [DOI] [PubMed] [Google Scholar]
- 12.Scirica BM, Moliterno DJ, Every NR, et al. Differences between men and women in the management of unstable angina pectoris (The GUARANTEE Registry). The GUARANTEE Investigators. Am J Cardiol. 1999;84(10):1145–1150. doi: 10.1016/S0002-9149(99)00525-1. [DOI] [PubMed] [Google Scholar]
- 13.Krumholz HM, Radford MJ, Ellerbeck EF, et al. Aspirin for secondary prevention after acute myocardial infarction in the elderly: prescribed use and outcomes. Ann Intern Med. 1996;124(3):292–298. doi: 10.7326/0003-4819-124-3-199602010-00002. [DOI] [PubMed] [Google Scholar]
- 14.Williams D, Bennett K, Feely J. Evidence for an age and gender bias in the secondary prevention of ischaemic heart disease in primary care. Br J Clin Pharmacol. 2003;55(6):604–608. doi: 10.1046/j.1365-2125.2003.01795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jha AK, Perlin JB, Steinman MA, Peabody JW, Ayanian JZ. Quality of ambulatory care for women and men in the Veterans Affairs Health Care System. J Gen Intern Med. 2005;20(8):762–765. doi: 10.1111/j.1525-1497.2005.0160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Persell SD, Maviglia SM, Bates DW, Ayanian JZ. Ambulatory hypercholesterolemia management in patients with atherosclerosis. Gender and race differences in processes and outcomes. J Gen Intern Med. 2005;20(2):123–130. doi: 10.1111/j.1525-1497.2005.40155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newby LK, LaPointe NM, Chen AY, et al. Long-term adherence to evidence-based secondary prevention therapies in coronary artery disease. Circulation. 2006;113(2):203–212. doi: 10.1161/CIRCULATIONAHA.105.505636. [DOI] [PubMed] [Google Scholar]
- 18.Stafford RS. Aspirin use is low among United States outpatients with coronary artery disease. Circulation. 2000;101(10):1097–1101. doi: 10.1161/01.cir.101.10.1097. [DOI] [PubMed] [Google Scholar]
- 19.Stafford RS, Monti V, Ma J. Underutilization of aspirin persists in US ambulatory care for the secondary and primary prevention of cardiovascular disease. PLoS Med. 2005;2(12):e353. doi: 10.1371/journal.pmed.0020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gornick ME, Eggers PW, Reilly TW, et al. Effects of race and income on mortality and use of services among Medicare beneficiaries. N Engl J Med. 1996;335(11):791–799. doi: 10.1056/NEJM199609123351106. [DOI] [PubMed] [Google Scholar]
- 21.Vaccarino V, Krumholz HM, Yarzebski J, Gore JM, Goldberg RJ. Sex differences in 2-year mortality after hospital discharge for myocardial infarction. Ann Intern Med. 2001;134(3):173–181. doi: 10.7326/0003-4819-134-3-200102060-00007. [DOI] [PubMed] [Google Scholar]
- 22.McCormick D, Gurwitz JH, Lessard D, Yarzebski J, Gore JM, Goldberg RJ. Use of aspirin, beta-blockers, and lipid-lowering medications before recurrent acute myocardial infarction: missed opportunities for prevention. Arch Intern Med. 1999;159(6):561–567. doi: 10.1001/archinte.159.6.561. [DOI] [PubMed] [Google Scholar]
- 23.Agency for Healthcare Research and Quality Rockville M. Puf Data Files. June 2003. Available from: http://www.meps.ahrq.gov/Puf/DataResultsData.asp. Accessed 2004–2005.
- 24.Cohen JW, Monheit AC, Beauregard KM, et al. The Medical Expenditure Panel Survey: a national health information resource. Inquiry. 1996;33(4):373–389. [PubMed] [Google Scholar]
- 25.Cohen SB. Design strategies and innovations in the medical expenditure panel survey. Med Care. 2003;41(7 Suppl):III5–III12. doi: 10.1097/01.MLR.0000076048.11549.71. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan PW, Morrato EH, Ghushchyan V, Wyatt HR, Hill JO. Obesity, inactivity, and the prevalence of diabetes and diabetes-related cardiovascular comorbidities in the U.S., 2000–2002. Diabetes Care. 2005;28(7):1599–1603. doi: 10.2337/diacare.28.7.1599. [DOI] [PubMed] [Google Scholar]
- 27.Correa-de-Araujo R, Miller GE, Banthin JS, Trinh Y. Gender differences in drug use and expenditures in a privately insured population of older adults. J Womens Health (Larchmt) 2005;14(1):73–81. doi: 10.1089/jwh.2005.14.73. [DOI] [PubMed] [Google Scholar]
- 28.Kass-Bartelmes BL, Altman BM, Taylor AK. Disparities and gender gaps in women’s health, 1996. Rockville (MD): Agency for Healthcare Research and Quality; 2001. MEPS Chartbook No. 8;AHRQ Pub. No. 02-0003.
- 29.Johansson S, Bergstrand R, Ulvenstam G, et al. Sex differences in preinfarction characteristics and longterm survival among patients with myocardial infarction. Am J Epidemiol. 1984;119(4):610–623. doi: 10.1093/oxfordjournals.aje.a113778. [DOI] [PubMed] [Google Scholar]
- 30.Mahon NG, McKenna CJ, Codd MB, O’Rorke C, McCann HA, Sugrue DD. Gender differences in the management and outcome of acute myocardial infarction in unselected patients in the thrombolytic era. Am J Cardiol. 2000;85(8):921–926. doi: 10.1016/S0002-9149(99)00902-9. [DOI] [PubMed] [Google Scholar]
- 31.Wenger NK. You’ve come a long way, baby: cardiovascular health and disease in women: problems and prospects. Circulation. 2004;109(5):558–560. doi: 10.1161/01.CIR.0000117292.19349.D0. [DOI] [PubMed] [Google Scholar]
- 32.Ayanian JZ. Increased mortality among middle-aged women after myocardial infarction: searching for mechanisms and solutions. Ann Intern Med. 2001;134(3):239–241. doi: 10.7326/0003-4819-134-3-200102060-00015. [DOI] [PubMed] [Google Scholar]
- 33.Ayanian JZ, Landrum MB, Guadagnoli E, Gaccione P. Specialty of ambulatory care physicians and mortality among elderly patients after myocardial infarction. N Engl J Med. 2002;347(21):1678–1686. doi: 10.1056/NEJMsa020080. [DOI] [PubMed] [Google Scholar]
- 34.Mulrow C, Pignone M. An editorial update: should she take aspirin. Ann Intern Med. 2005;142(11):942–943. doi: 10.7326/0003-4819-142-11-200506070-00015. [DOI] [PubMed] [Google Scholar]
- 35.Tran C, Knowles SR, Liu BA, Shear NH. Gender differences in adverse drug reactions. J Clin Pharmacol. 1998;38(11):1003–1009. doi: 10.1177/009127009803801103. [DOI] [PubMed] [Google Scholar]
- 36.Martin RM, Biswas PN, Freemantle SN, Pearce GL, Mann RD. Age and sex distribution of suspected adverse drug reactions to newly marketed drugs in general practice in England: analysis of 48 cohort studies. Br J Clin Pharmacol. 1998;46(5):505–511. doi: 10.1046/j.1365-2125.1998.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamada T, Alpers D, Kaplowitz N, Loren L, Owyang C, Powel D. Textbook of gastroenterology. 4th ed: Lippincott Williams & Wilkins; 2003.
- 38.Center for Disease Control and Prevention. National Center for Health Statistics. Health Data for All Ages. Available from: http://www.cdc.gov/nchs/healthdataforallages.htm. Accessed November 21st, 2005.
- 39.Sam C, Massaro JM, D’Agostino RB, Sr, et al. Warfarin and aspirin use and the predictors of major bleeding complications in atrial fibrillation (the Framingham Heart Study) Am J Cardiol. 2004;94(7):947–951. doi: 10.1016/j.amjcard.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 40.Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med 1989;321(3):129–35. [DOI] [PubMed]
- 41.de Marco R, Locatelli F, Sunyer J, Burney P. Differences in incidence of reported asthma related to age in men and women. A retrospective analysis of the data of the European Respiratory Health Survey. Am J Respir Crit Care Med. 2000;162(1):68–74. doi: 10.1164/ajrccm.162.1.9907008. [DOI] [PubMed] [Google Scholar]
- 42.Gollapudi RR, Teirstein PS, Stevenson DD, Simon RA. Aspirin sensitivity: implications for patients with coronary artery disease. JAMA. 2004;292(24):3017–3023. doi: 10.1001/jama.292.24.3017. [DOI] [PubMed] [Google Scholar]
- 43.Hurlen M, Abdelnoor M, Smith P, Erikssen J, Arnesen H. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002;347(13):969–974. doi: 10.1056/NEJMoa020496. [DOI] [PubMed] [Google Scholar]
- 44.Hurlen M, Eikvar L, Seljeflot I, Arnesen H. Occult bleeding in three different antithrombotic regimes after myocardial infarction A WARIS-II subgroup analysis. Thromb Res. 2006;118(4):433–438. doi: 10.1016/j.thromres.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 45.Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 46.Kriegsman DM, Penninx BW, van Eijk JT, Boeke AJ, Deeg DJ. Self-reports and general practitioner information on the presence of chronic diseases in community dwelling elderly. A study on the accuracy of patients’ self-reports and on determinants of inaccuracy. J Clin Epidemiol. 1996;49(12):1407–1417. doi: 10.1016/S0895-4356(96)00274-0. [DOI] [PubMed] [Google Scholar]
- 47.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57(10):1096–1103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Bowlin SJ, Morrill BD, Nafziger AN, Lewis C, Pearson TA. Reliability and changes in validity of self-reported cardiovascular disease risk factors using dual response: the behavioral risk factor survey. J Clin Epidemiol. 1996;49(5):511–517. doi: 10.1016/0895-4356(96)00010-8. [DOI] [PubMed] [Google Scholar]
- 49.Bowlin SJ, Morrill BD, Nafziger AN, Jenkins PL, Lewis C, Pearson TA. Validity of cardiovascular disease risk factors assessed by telephone survey: the Behavioral Risk Factor Survey. J Clin Epidemiol. 1993;46(6):561–571. doi: 10.1016/0895-4356(93)90129-O. [DOI] [PubMed] [Google Scholar]
- 50.Bergmann MM, Byers T, Freedman DS, Mokdad A. Validity of self-reported diagnoses leading to hospitalization: a comparison of self-reports with hospital records in a prospective study of American adults. Am J Epidemiol. 1998;147(10):969–977. doi: 10.1093/oxfordjournals.aje.a009387. [DOI] [PubMed] [Google Scholar]
- 51.Martin LM, Leff M, Calonge N, Garrett C, Nelson DE. Validation of self-reported chronic conditions and health services in a managed care population. Am J Prev Med. 2000;18(3):215–218. doi: 10.1016/S0749-3797(99)00158-0. [DOI] [PubMed] [Google Scholar]
- 52.Kulkarni SP, Alexander KP, Lytle B, Heiss G, Peterson ED. Long-term adherence with cardiovascular drug regimens. Am Heart J. 2006;151(1):185–191. doi: 10.1016/j.ahj.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 53.Balkrishnan R. Predictors of medication adherence in the elderly. Clin Ther. 1998;20(4):764–771. doi: 10.1016/S0149-2918(98)80139-2. [DOI] [PubMed] [Google Scholar]
- 54.DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42(3):200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 55.Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38(2):303–312. doi: 10.1345/aph.1D252. [DOI] [PubMed] [Google Scholar]