Abstract
Fusarium head blight (FHB) of wheat, caused by Fusarium graminearum and other Fusarium species, is a major disease problem for wheat production worldwide. To combat this problem, large-scale breeding efforts have been established. Although progress has been made through standard breeding approaches, the level of resistance attained is insufficient to withstand epidemic conditions. Genetic engineering provides an alternative approach to enhance the level of resistance. Many defense response genes are induced in wheat during F. graminearum infection and may play a role in reducing FHB. The objectives of this study were (1) to develop transgenic wheat overexpressing the defense response genes α-1-purothionin, thaumatin-like protein 1 (tlp-1), and β-1,3-glucanase; and (2) to test the resultant transgenic wheat lines against F. graminearum infection under greenhouse and field conditions. Using the wheat cultivar Bobwhite, we developed one, two, and four lines carrying the α-1-purothionin, tlp-1, and β-1,3-glucanase transgenes, respectively, that had statistically significant reductions in FHB severity in greenhouse evaluations. We tested these seven transgenic lines under field conditions for percent FHB disease severity, deoxynivalenol (DON) mycotoxin accumulation, and percent visually scabby kernels (VSK). Six of the seven lines differed from the nontransgenic parental Bobwhite line for at least one of the disease traits. A β-1,3-glucanase transgenic line had enhanced resistance, showing lower FHB severity, DON concentration, and percent VSK compared to Bobwhite. Taken together, the results showed that overexpression of defense response genes in wheat could enhance the FHB resistance in both greenhouse and field conditions.
Keywords: Fusarium head blight, Wheat, Fusarium graminearum, Transgenic wheat, Triticum aestivum
Introduction
Fusarium head blight (FHB; scab), principally caused by Fusarium graminearum Schwabe (teleomorph Gibberella zeae (Schwein.) Petch), is a devastating disease of wheat (Triticum aestivum L.) and barley (Hordeum vulgare L.) throughout the world (Sutton 1982; McMullen et al. 1997). Between 1993 and 2001, in United States, an estimated US$ 8 billion loss was incurred from FHB (Nganje et al. 2004). The disease is favored by warm conditions with frequent rainfall and high humidity during flowering. Yield reductions result from reduction in the kernel number and the presence of dry, shriveled kernels, often referred to as ‘tombstone kernels’. Additionally, reductions in grain quality are caused by Fusarium trichothecene mycotoxins such as deoxynivalenol (DON; Bai and Shaner 1994; Sutton, 1982; Tuite et al. 1990).
The most economical and efficient way to protect wheat from FHB is to develop genetically-resistant varieties. Wheat breeding programs are utilizing resistance to initial infection (Type I) and resistance to spread of the disease (Type II) as the primary forms of resistance (Rudd et al. 2001). However, these forms of resistance are partial (Kolb et al. 2001), and the level of genetic resistance provided is generally insufficient to withstand a FHB epidemic. Therefore, novel sources of resistance are required, and genetic engineering is one approach to develop novel resistance in wheat.
Several classes of genes have the potential to provide genetically-engineered resistance to FHB in wheat. One group of genes, referred to as pathogenesis-related (PR) or defense response genes, encode proteins such as β-1,3-glucanases, chitinases, thaumatin-like proteins (tlps) and thionins whose expression often increase as part of the plant host defense response to pathogen attack (Linthorst 1991). Many defense response genes were shown to be induced in wheat (Pritsch et al. 2000, 2001; Li et al. 2001; Kang and Buchenauer 2002; Kong et al. 2005; Han et al. 2005; Zhou et al. 2005; Bernardo et al. 2006) and barley (Boddu et al. 2006) spikes during F. graminearum infection. In particular, PR1, PR-2 (β-1,3-glucanase), PR-3 (chitinase), PR-4, and PR-5 (tlp-1) transcripts accumulated in wheat spikes during F. graminearum infection (Pritsch et al. 2000, 2001). In addition, polyphenol oxidase activities were detected in resistant wheat genotypes (Mohammadi and Kazemi 2002). Furthermore, Kang and Buchenauer (2003) showed accumulation of thionin proteins in F. culmorum-infected wheat tissues. These findings demonstrated that wheat and barley mount an induced defense response to Fusarium infection that involves many defense response genes.
Overexpression of defense response genes in transgenic plants has provided enhanced resistance to a variety of fungal pathogens (Muehlbauer and Bushnell 2003). For example, transgenic wheat lines carrying a barley-seed class II chitinase exhibited enhanced resistance to powdery mildew (Bliffeld et al. 1999; Oldach et al. 2001). Varying amounts of resistance towards powdery mildew were observed in transgenic wheat lines carrying a barley chitinase or a barley β-1,3-glucanase (Bieri et al. 2003). With respect to FHB, a transgenic wheat line carrying a rice tlp and a line carrying a combination of a wheat β-1,3-glucanase and chitinase exhibited delayed symptoms of FHB in greenhouse trials (Chen et al. 1999; Anand et al. 2003). However, neither transgenic wheat line exhibited any resistance to FHB under field conditions (Anand et al. 2003). In addition, transgenic Arabidopsis carrying an overexpressed Arabidopsis thionin showed increased resistance to F. oxysporum (Epple et al. 1997). Recently, transgenic wheat expressing the Arabidopsis NPR1 gene, a gene that regulates defense responses, was shown to exhibit a high level of resistance to FHB in greenhouse evaluations (Makandar et al. 2006).
As part of our effort to increase variation for genetic resistance to FHB and to understand the relationship between defense response gene expression and FHB resistance, we produced wheat plants carrying a wheat α-1-purothionin, a barley tlp-1, or a barley β-1,3-glucanase transgene. We evaluated these plants against FHB under greenhouse and field conditions. Our results show that the overexpression of α-1-purothionin, tlp-1, or β-1,3-glucanase in wheat results in enhanced resistance to FHB.
Materials and methods
Plant materials
The spring wheat cultivars ‘Wheaton’, ‘Roblin’, ‘Alsen’, ‘2375’, ‘Sumai 3’, and ‘Bobwhite’ were used as checks for FHB responses. Wheaton and Roblin are hard red spring wheat cultivars that are highly susceptible to FHB; Bobwhite is a cultivar from CIMMYT that is susceptible to FHB; 2375 is moderately susceptible to FHB; Alsen is moderately resistant to FHB with resistance derived from Sumai 3; and Sumai 3 is a Chinese cultivar known for resistance to spread of disease in the spike (Type II resistance; Bai and Shaner 1996). The cultivar Bobwhite was used as parental material for transformations.
Plant transformation plasmids
pAHC25
The pAHC25 plasmid (Fig. 1; Christensen et al. 1992), containing the uidA and bar genes under the control of the maize ubiquitin promoter, was kindly donated by Dr. Peter Quail of the Plant Gene Expression Center, University of California at Berkeley. The uidA gene encodes β-glucuronidase and the bar gene encodes the enzyme phosphinothricin acetyltransferase which confers resistance to the phosphinothricin-containing herbicides.
Fig. 1.
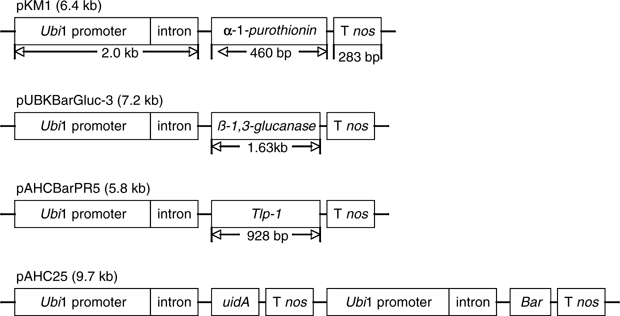
Plasmids used for wheat transformation. Plasmids containing the wheat α-1-purothionin (pKM1), barley tlp-1 transgene (pAHCBarPR5), and barley β-1,3-glucanase (pUBKBarGluc-3) were co-bombarded with pAHC25 to develop transgenic wheat plants. The ubiquitin 1 promoter and intron is from the maize ubiquitin gene, and the T nos termination sequence is from the nopaline synthase gene from Agrobacterium tumefaciens. The uidA gene encodes β-glucuronidase and is from Escherichia coli and the bar gene encodes the enzyme phosphinothricin acetyltransferase and is from Streptomyces hygroscopicus
pKM1
A plasmid containing a 460 bp wheat α-1-purothionin gene (GenBank accession number X70665.1) under the control of the maize ubiquitin promoter was kindly provided by Dr. Ann Blechl (USDA-ARS, Albany, CA). The α-1-purothionin gene was cloned into the BamHI/BglII site (replacement of the bar gene) of pUBK BglII−. The pUBK BglII− vector, kindly provided by Drs. Ann Blechl, Pat Okubara, and Kent McCue (USDA-ARS, Albany, CA), is a derivative of the pAHC20 vector (Christensen et al. 1992). pUBK BglII− contains the ubiquitin promoter, with the BglII site removed, driving the bar gene.
pAHCBarPR5
Barley tlp-1 cDNA (GenBank accession number AM403331) was removed from the parent plasmid (kindly provided by Dr. David Collinge, Department of Plant Biology, Royal Veterinary and Agricultural University, Denmark) by XhoI digestion, blunt-end repaired and cloned into the blunt-end repaired BamHI site of pAHC17. The expression cassette in the pAHC17 vector (Christensen et al. 1992) contains the maize ubiquitin promoter and the NOS terminator element. The pAHC17 vector was kindly provided by Dr. Peter Quail of the Plant Gene Expression Center, University of California at Berkeley.
pUBKBarGluc-3
The 1234 bp barley class-II β-1,3-glucanase cDNA (GenBank accession number M62907.1; Leah et al. 1991) was removed from the parent plasmid (kindly provided by Dr. John Mundy, Carlsberg Research Laboratory, Copenhagen, Denmark) by EcoRI digestion, blunt-end repaired and ligated into the blunt-end repaired BglII/BamHI site (replacement of bar gene) of the expression vector pUBK BglII−.
Wheat transformation
Spring wheat (cv. Bobwhite) was used for all transformations. Particle gun bombardment of embryos, selection, and regeneration were carried out as described by Mackintosh et al. (2006). We conducted cotransformation of pAHC25 with pKM1, pAHCBarPR5, or pUBKBarGluc-3.
RNA isolation and transcript analysis
RNA was isolated from leaf tissue using the Trizol reagent (Invitrogen, Carlsbad, CA) as per manufacturers’ instructions. RNA was subjected to RT-PCR based on the protocol accompanying the Calypso RT-PCR kit (GenSys Ltd., Farnborough, UK) using primers synthesized by Integrated DNA Technologies Inc. (Coralville, IA). The 5′ sense primer was a maize ubiquitin promoter sequence (5′-GATGCATATACATGATGGCATATGCAG-3′) and the 3′ antisense primers were oligonucleotides that corresponded to defense response gene coding sequences for α-1-purothionin (5′-GTTACAGAAATTGACACAAGCATCGCC-3′), tlp-1 (5′-GACAGAAGGTGATCTGGTAGTTATTATT-3′) and β-1,3-glucanase (5′-GATGTTCACGGCAGGGTAGT-3′ and 5′-GCCACGTCCGTCATGTAGGCGTTC-3′). A wheat actin gene (GenBank accession number AB181991) with the primer sequences 5′-GCCACACTGTTCCAATCTATGA-3′ and 5′-TGATGGAATTGTATGTCGCTTC-3’ was used as a positive control. Sizes for the amplified products from the α-1-purothionin and tlp-1 transgenes were 600 and 805, respectively. Sizes for the two primers for the β-1,3-glucanase transgene were 577 and 777 bp. Size for the wheat actin gene was 369 bp.
Greenhouse evaluation of transgenic lines against Fusarium graminearum infection
Seeds of each transgenic line were planted in the greenhouse. At anthesis, one spikelet at the central node of the main spike of each plant was inoculated with 10 μl of a macro-conidial spore suspension (100,000 conidia/ml) of Fusarium graminearum isolate Butte 86ADA-11 (Evans et al. 2000; NRRL 38661). Plants were placed in a dew chamber for 72 h following inoculation and then returned to the greenhouse. Disease severity was assessed at 20 days after inoculation by counting the number of infected spikelets and expressing the infection level as a percentage of the total number of spikelets for each spike. Bobwhite, Wheaton, and Sumai 3 were used as checks in each greenhouse test.
Field evaluation of transgenic lines against Fusarium graminearum infection
Transgenic wheat lines were examined for their reaction to FHB in the field. RT-PCR positive plants of each of the lines were selected as the source of seed for the field plantings. In addition to the test lines, Bobwhite was included as the untransformed control. Two experiments were conducted during the summers of 2004 and 2005 at the University of Minnesota Agricultural Experiment Station, Crookston, Minnesota. T6 and T7 of the transgenic lines were used for the 2004 and 2005 field tests, respectively. The field tests were each a randomized complete block design with four replications. Entries were established in two-row plots; rows were 2.4 m (8 ft) long and were spaced 0.3 m (1 ft) apart. Within rows, seed was planted at a rate of 3.3 g of seed/m. Alsen, 2375, Roblin and Wheaton were also included in the experiment as disease response checks. Additional plantings of noninoculated Wheaton were included in the field trial to determine the level of disease.
Inoculum consisted of a mixture of 12 isolates of F. graminearum. These came from naturally infected samples of grain from commercial fields of wheat and barley in Minnesota from 2002 to 2004. Plots were inoculated twice; the first time at anthesis and then 3 days later. Inoculum (1 × 105 macroconidia/ml) was applied at a rate of 33 ml/m of row using a CO2-powered backpack sprayer, at a pressure of 276 kPa and fitted with a flat-fan spray tip (TeeJet SS8003, Spraying Systems Co., Wheaton, IL).
FHB incidence and severity were evaluated visually 21 days after the initial inoculation. Incidence was recorded as the percentage of spikes with symptomatic spikelets and severity as the percentage of symptomatic spikelets in 20 spikes of primary tillers arbitrarily selected per plot.
Plots were harvested with a Wintersteiger classic combine (Wintersteiger, Ried, Austria) at maturity. The percentage of visually scabby kernels (VSK) was assessed on a hand-cleaned 50 g sample by comparison to standards with a known percentage of scabby kernels according to the procedure of Jones and Mirocha (1999).
Following VSK analysis, the samples were ground for 2 min with a Stein Laboratory Mill (model M-2, Stein Laboratories, Atchison, KS) and analyzed for deoxynivalenol (DON) concentration using gas chromatography and mass spectrometry according to the procedures of Mirocha et al. (1998) with the following modifications. DON was extracted from 4 g of the ground wheat placed in a 50 ml centrifuge tube to which 16 ml of acetonitrile:water (84:16 v/v) was added. Samples were derivatized using 100 μl of the silylating reagent (TMSI/TMCS, 100:1), 1 ml of isooctane and 1 ml of distilled water.
Western blot analysis
Spike tissue was ground using liquid nitrogen and protein was extracted by vortexing the tissue at 4°C for 10 min in extraction buffer (50 mM NaH2PO4, pH 6.8, 100 mM PMSF). After micro-centrifugation at 4°C, full speed, for 5 min, supernatant protein measurements were conducted using Biorad reagent (Biorad) with bovine serum albumin as a standard. Extracts containing 10 μg protein were used to determine the amount of transgenic protein present in transgenic lines using Western blotting.
Samples were subjected to SDS-PAGE using 12% gels, transferred to PVDF-PLUS transfer membrane (Micron Separations Inc., Westborough, MA) and cross reacted with an affinity-purified polyclonal antibody (1:1000 dilution of supplied material). The tlp-1 and β-1,3-glucanase antibodies were provided by Quality Controlled Biochemicals Inc., Hopkinton, MA. For tlp-1, two peptides (QAYQHPNDVATHAC and CINVPAG TQAGRIWAR) were used to raise the antibody. For β-1,3-glucanase, one peptide (CGLFNPDKSPAYNIQF) was used to raise the antibody. Protein was visualized using an ECF Western Blotting Reagent Pack (rabbit) (Amersham Biosciences, Piscataway, NJ), and fluorescence detection was carried out using a Storm 840 (Molecular Dynamics, Sunnyvale, CA). Specificity of β-1,3-glucanase and tlp-1 antibodies was confirmed through cross-reacting the antibodies with the peptides on Western blots.
Southern blot analysis
DNA isolation, gel electrophoresis, gel blotting, hybridization, and washing were conducted according to de la Peña et al. (1996). Radio-labeled probes for tlp-1, β-1,3-glucanase, and α-1-purothionin were used in the hybridization reactions. The subsequent banding patterns were visualized using autoradiography.
Statistical analysis
For the greenhouse evaluation data, t-tests were used to compare each transgenic line to the parental Bobwhite controls. For the field evaluation data from 2004 and 2005, all analyses were performed with SAS® Version 9.1 (SAS Institute, Cary, NC). Analyses of variance were performed using PROC MIXED procedure. The statistical model included genotype, year and genotype-by-year interactions as fixed factors, and replication nested within year as a random factor was used as an error term for testing year effect. For each experiment, homogeneity of variances among genotypes was checked for each trait using PROC UNIVARIATE. For each trait, when variances were found to be more than four times different from each other A REPEATED/GROUP = statement of PROC MIXED was used to account for the heterogeneity of variances. Least square means and pairwise comparisons between means were obtained using LSMEANS and PDIFF options.
Results
Generation of transgenic wheat plants
The wheat cultivar Bobwhite was used as parental material for all transformation experiments. The pAHC25 plasmid and either the pKM1 (wheat α-1-purothionin), pAHCBarPR5 (barley tlp-1), or pUBKBarGluc-3 (barley β-1,3-glucanase) plasmids were used in cotransformation experiments. Figure 1 shows a schematic of each plasmid. The correct orientation within the vector and open reading frame integrity of the inserted cDNA in pKM1, pAHCBarPR-5, and pUBKBarGluc-3 was confirmed by DNA sequence analysis. pAHC25 carries the uidA gene for visual scoring of β-glucuronidase (GUS) activity and the bar gene which confers tolerance to the herbicide phosphinothricin for selection. Both the uidA and bar genes were driven by the promoter from the maize ubiquitin gene. Selection and regeneration of plants was conducted as described in Mackintosh et al. (2006).
To identify transgenic wheat plants carrying the α-1-purothionin, barley tlp-1 and barley β-1,3-glucanase transgenes, we conducted RT-PCR analysis on the T0 plants. We identified 25, 25, and 31 transgenic wheat lines carrying expressed wheat α-1-purothionin, barley tlp-1, and barley β-1,3-glucanase, respectively. Table 1 shows the number of embryos bombarded for each plasmid, and the percent transformed plants carrying the expressed transgene of interest. Our efficiency for recovering transgenic wheat plants expressing the transgenes of interest ranged from 1.4 to 3%.
Table 1.
Production of transgenic wheat plants
| Transgene | Number of embryos bombarded | Number of plants expressing transgenea | Transformation (%) |
|---|---|---|---|
| Wheat α-1-purothionin | 1787 | 25 | 1.4 |
| Barley thaumatin-like protein-1 | 825 | 25 | 3.0 |
| Barley β-1,3-glucanase | 1079 | 31 | 2.9 |
aExpression based on RT-PCR of each transgene.
To obtain T2 lines for further characterization, we grew five T1 seeds from each T0 plant. Each T1 plant was tested by RT-PCR for expression of the appropriate transgene, and T2 seed was collected from plants expressing each transgene.
Greenhouse evaluation of transgenic plants for response to Fusarium head blight
To identify transgenic lines with enhanced resistance to FHB and to eliminate susceptible lines, we conducted two greenhouse evaluations for FHB resistance. Of the 81 transgenic wheat lines developed, 70 (18 of 25 wheat α-1-purothionin, 23 of 25 barley tlp-1 and 29 of 31 barley β-1,3-glucanase) lines produced enough T2 seed for FHB evaluations. Sixteen to 20 seeds were planted for each line and inoculated with F. graminearum. We assayed the spread of the disease following point inoculation, and analyzed the results as the percent disease severity at 20 days after inoculation. In addition, each plant in the α-1-purothionin lines was assayed for transgene expression using RT-PCR. Only those plants expressing the α-1-purothionin transgene were used to evaluate the efficacy of α-1-purothionin against FHB. The plants carrying the tlp-1 and β-1,3-glucanase transgenes were not assayed for transgene expression in the initial T2 FHB screen. For the lines carrying the tlp-1 and β-1,3-glucanase transgenes, data from all plants assayed for FHB resistance, which would have included transgene null together with transgene homozygous and hemizygous plants, were used to calculate the percent FHB severity. We compared FHB severity in the transgenic lines against the nontransformed Bobwhite parent.
Based on this initial experiment, we eliminated the most susceptible lines (>50% disease severity) and reevaluated 6, 13, and 16 T2 lines carrying the α-1-purothionin, tlp-1, and β-1,3-glucanase transgenes, respectively. We also evaluated the T3 lines of the same 6 lines carrying the α-1-purothionin transgene, and 13 lines carrying the tlp-1 transgene. Again, we planted 16–20 plants per line, and evaluated the lines against FHB. In this screen, all plants were assayed for transgene expression. Only those plants expressing the transgene were used to calculate disease severity for comparison against the nontransformed Bobwhite.
From the initial disease screens on the 70 transgenic lines, we identified seven lines with enhanced FHB resistance. One line had the α-1-purothionin transgene and is referred to as CM 17, two lines carried the tlp-1 transgene and are referred to as CM21 and CM23, and four lines had barley β-1,3-glucanase transgene and are referred to as CM27, CM28, CM30, and CM33. The results for these initial two screens of the resistant transgenic lines are shown in Table 2. The resistant transgenic lines were evaluated in further FHB disease screens in the greenhouse (Table 2). For these additional greenhouse evaluations, only plants expressing the transgene, based on RT-PCR assays, were used to calculate the percent FHB severity. One line carrying the α-1-purothionin transgene (CM17) significantly reduced FHB severity in four of five screens (P < 0.05), and had an overall average reduction of 34%. The tlp-1 transgenic CM23 and CM21 lines significantly reduced FHB severity when compared to the Bobwhite control in three of four or five screens, respectively (P < 0.05). Taking the average disease severity over all screen replicates, CM21 and CM23 reduced disease severity compared to Bobwhite by 30% and 36%, respectively. The ß-1,3-glucanase transgenic CM27, CM28, CM30 and CM33 lines significantly reduced FHB severity compared to the Bobwhite control in two to three screens (P < 0.05). The average reduction in disease severity compared to Bobwhite for CM27, CM28, CM30, and CM33 was 40, 49, 47, and 38%, respectively. While all seven transgenic lines had similar levels of enhanced disease resistance, the lines with the β-1,3-glucanase transgene had slightly better disease control.
Table 2.
Percent Fusarium head blight severity in greenhouse evaluations of seven wheat lines carrying wheat α-1-purothionin, barley thaumatin-like protein 1, or barley β-1,3-glucanase that were selected in initial tests and three wheat varieties used as disease checks
| Generation testeda | ||||||||
|---|---|---|---|---|---|---|---|---|
| Genotypeb | T2 | T2 | T2 | T3 | T3 | T3 | T3 | T4 |
| CM17 | 38*(9) | 44**(8) | –c | 38**(10) | 74 (14) | - | - | 28* (22) |
| CM21 | 39*(12) | 38 (4) | 26*** (11) | 89 (11) | – | – | 57* (22) | – |
| CM23 | 37*(14) | 55 (14) | 44** (18) | 41*(12) | – | – | – | – |
| CM27 | 38*(12) | 34**(12) | – | – | 55* (17) | – | – | – |
| CM28 | 26**(10) | 37*(10) | – | – | 41** (12) | – | – | – |
| CM30 | 19***(8) | 36*(11) | – | – | 51 (4) | – | – | – |
| CM33 | 33 (8) | 48*(11) | 44* (7) | – | – | 40* (12) | – | – |
| Bobwhite | 63 (31) | 71 (28) | 78 (28) | 71 (28) | 78 (28) | 64 (18) | 73 (33) | 54 (36) |
| Wheaton | 70 (33) | 85 (45) | – | 85 (45) | – | 91 (21) | 94 (57) | 99 (60) |
| Sumai 3 | 26 (22) | 9 (25) | 10 (78) | 9 (25) | 10 (78) | 21 (16) | 16 (46) | 7 (61) |
Numbers in parenthesis represent the number of plants in the screen.
aIndicates the generation that was evaluated. T2 and T3 lines that were evaluated in the initial FHB disease screens are in bold. Each column, except for the second T2 screen and the first T3 screen, represent individual experiments where lines were evaluated simultaneously.
bCM17 is a transgenic wheat line carrying the wheat α-1-purothionin, CM21 and CM23 are transgenic wheat lines carrying barley thaumatin-like protein 1, and CM27, CM28, CM30, and CM33 are the transgenic wheat lines carrying barley β-1,3-glucanase transgene. Bobwhite is the variety transformed and susceptible check, Wheaton is a susceptible check, and Sumai 3 is a resistant check. It is not known whether the transgenic lines were homozygous for the transgene or segregating.
cIndicates that this line was not examined in this screen.
*Significance at the 0.05 compared to Bobwhite.
**Significance at the 0.01 compared to Bobwhite.
***Significance at the 0.001 level compared to Bobwhite.
Molecular characterization of transgenic plants
To verify that one, two, and four lines transformed with α-1-purothionin, tlp-1, and β-1,3-glucanase transgenes, respectively, were transgenic, we conducted Southern blot analysis. Our Southern blots also provided the opportunity to determine if the two tlp-1 lines and four β-1,3-glucanase lines were independent events. Genomic DNA was isolated from each line, digested with the appropriate restriction enzyme, blotted, and hybridized with a radio-labeled probe from the α-1-purothionin, tlp-1, or β-1,3-glucanase transgenes (Fig. 2). Each transgenic line contained at least one unique band compared to the nontransformed Bobwhite, indicating that each of the lines were transgenic for the appropriate transgene. In addition, the banding patterns of the two tlp-1 lines and the four β-1,3-glucanase lines were distinct, indicating that the two tlp-1 lines were independent events, and the four β-1,3-glucanase lines were also independent events.
Fig. 2.
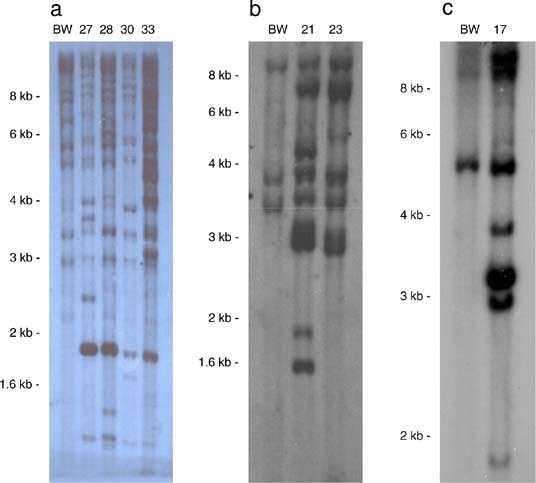
a–c Southern blot analysis of transgenic wheat plants. aEcoRI-digested genomic DNA from untransformed Bobwhite (BW), and pUBKBarGluc-3 transgenic CM27, CM28, CM30, and CM33 plants hybridized with a probe designed to bridge the ubiquitin promoter and the β-1,3-glucanase transgene junction. bHindIII-digested genomic DNA from untransformed Bobwhite (BW), and pAHCBarPR5 transgenic CM21 and CM23 plants and hybridized with a probe designed to bridge the ubiquitin promoter and the tlp-1 transgene junction. cXhoI-digested genomic DNA from untransformed Bobwhite (BW), and pKM1 transgenic CM17 plants hybridized with a probe designed to bridge the ubiquitin promoter and the α-1-purothionin transgene junction
To confirm transgene expression, we conducted RT-PCR and Western blot analyses. As stated earlier, RT-PCR was conducted on each plant used in the greenhouse disease evaluations, except where indicated. Figure 3 shows an example of the RT-PCR analysis of the lines carrying each transgene. We also conducted Western blot analysis on plants carrying the tlp-1, and β-1,3-glucanase transgenes. We isolated protein from spikes, blotted the protein, and cross-reacted the blots with antibodies specific for tlp-1 and β-1,3-glucanase proteins. Our results showed that the transgenic lines exhibited an increase in their appropriate transgene protein compared to the nontransgenic Bobwhite control (Fig. 4).
Fig. 3.
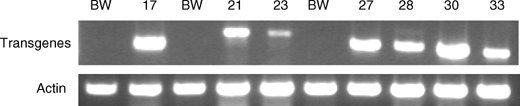
RT-PCR analysis of transgenic wheat lines carrying the wheat α-1-purothionin (CM17), barley tlp-1 (CM21 and CM 23), and barley β-1,3-glucanase (CM27, CM28, CM30, and CM33) transgenes. The fragment sizes for the α-1-purothionin, barley tlp-1, and barley β-1,3-glucanase amplified the expected products of 600, 805, and 577 bp, respectively. The wheat actin gene was used as a positive control and it exhibited the expected size of 369 bp
Fig. 4.
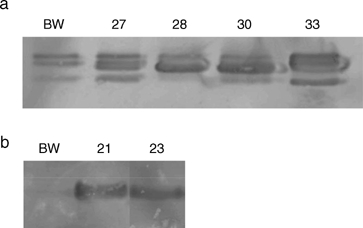
a–b Western blot analysis of transgenic wheat lines. a Protein extracted from spikes of transgenic lines carrying barley β-1,3-glucanase (CM27, CM28, CM30, and CM33) transgene was subjected to SDS-PAGE using a 12% polyacrylamide gel. Our barley β-1,3-glucanase antibody does not distinguish the transgenic barley protein from the endogenous wheat protein. The transgene-specific protein band in line CM33 appears to exhibit a higher molecular weight. Molecular markers indicated the protein to be the expected 35.2 kDa size. b Protein extracted from spikes of lines carrying barley tlp-1 (CM21 and CM23) transgene was subjected to SDS-PAGE using a 10% polyacrylamide gel. Molecular markers indicated the protein to be the expected 17.5 kDa size
Field evaluation of transgenic plants for response to Fusarium head blight
To further examine the level of effect on FHB, we conducted field tests of these seven lines in the summers of 2004 and 2005. Seed for each of the seven lines was derived from plants expressing the transgene based on RT-PCR analysis. We scored the lines for percent FHB severity, DON concentration, and percent visually scabby kernels (VSK) and compared the lines to the parental cultivar Bobwhite (Table 3). All transgenic lines tested in the field except for CM28 showed a significant reduction in at least one FHB disease measure in comparison with Bobwhite. Four, four, and two lines exhibited significant reductions in percent FHB severity, DON concentration, and percent VSK, respectively. Although CM30 showed a significant reduction in percent FHB severity, it showed a significant increase in DON concentration. CM27 was the only transgenic line that exhibited a significant reduction in all three disease measures.
Table 3.
Percent Fusarium head blight (FHB) severity, deoxynivalenol (DON) concentration, and percent visual scabby kernels (VSK) in transgenic wheat plants carrying wheat α-1-purothionin, barley thaumatin-like protein l, and barley β-1,3-glucanase and check wheat varieties evaluated in the field in 2004 and 2005
| Genotypea | FHB severity (%) | DON concentration (ppm)b | VSK (%) |
|---|---|---|---|
| Bobwhite | 65.1 | 16.3 | 29.6 |
| Alsen | 15.4*** | 3.7*** | 5.4*** |
| Wheaton | 81.2*** | 26.2*** | 51.9*** |
| Wheaton (noninoculated) | 64.3 | 17.8 | 32.2 |
| Roblin | 70.9 | 18.8 | 42.2* |
| 2375 | 46.2** | 8.3*** | 11.6*** |
| CM17 | 52.7* | 15.7 | 24.7 |
| CM21 | 55.1 | 11.4*** | 19.8* |
| CM23 | 57.2 | 13.4* | 21.0 |
| CM27 | 46.5*** | 9.9*** | 17.7* |
| CM28 | 58.2 | 17.6 | 25.8 |
| CM30 | 48.3*** | 22.8** | 34.3 |
| CM33 | 49.2*** | 14.3 | 20.3 |
aCM17 is a transgenic wheat line carrying the wheat α-1-purothionin, CM21 and CM23 are the transgenic wheat lines carrying barley thaumatin-like protein 1, and CM27, CM28, CM30, and CM33 are the transgenic wheat lines carrying barley β-1,3-glucanase transgene. T6 and T7 were used for the 2004 and 2005 field screens, respectively. Bobwhite is the variety transformed and susceptible check, Wheaton and Roblin are the susceptible checks, 2375 is a moderately resistant check, and Alsen is a resistant check.
bParts per million deoxynivalenol concentration.
*Significance at the 0.05 level compared to Bobwhite.
**Significance at the 0.01 level compared to Bobwhite.
***Significance at the 0.001 level compared to Bobwhite.
Discussion
Large-scale wheat breeding efforts have not resulted in the development of highly resistant varieties to FHB. This is due to the fact that resistance in wheat is partial and quantitative. That is, multiple loci in wheat explain just a portion of the variation for FHB resistance (e.g., Kolb et al. 2001). Single genes conferring a high degree of resistance to FHB have not been found despite extensive searches of wheat germplasm resources (Leonard and Bushnell 2003). One characteristic of the wheat response to F. graminearum infection is the induction of defense response genes such as β-1,3-glucanase, tlp-1, and thionin genes (Chen et al. 1999; Pritsch et al. 2000, 2001; Li et al. 2001; Kang and Buchenauer 2002; Han et al. 2005; Zhou et al. 2005; Bernardo et al. 2006). These genes are thought to provide basal resistance during infection because they encode proteins with differing modes of action against fungal pathogens. Thionins and tlps damage fungal cell membranes by making them permeable (Bohlmann et al. 1988; Yun et al. 1998), whereas β-1,3-glucanases degrade cell wall polysaccharide linkages (Leah et al. 1991). In this study, we produced transgenic wheat lines overexpressing either α-1-purothionin, a tlp-1, or a β-1,3-glucanase to test their efficacy against FHB.
Numerous studies reveal that over-expression of defense response genes in transgenic plants results in enhanced resistance to various fungal pathogens (reviewed in Muehlbauer and Bushnell 2003). In general, these studies show that partial resistance can be achieved from over-expressing defense response genes in plants. In this study, we defined enhanced resistance as exhibiting a reduction in any of the three disease parameters. To date, there are no reports of commercially practical levels of fungal resistance derived from over-expressing defense response genes.
From our initial 70 transgenic lines, there were seven lines carrying either α-1-purothionin, tlp-1, or β-1,3-glucanase transgenes that resulted in enhanced FHB resistance in the greenhouse. Enhanced resistance was not detected in each of these seven lines in every greenhouse screen conducted (Table 2). These results are likely due to the high variability inherent in FHB disease screens. However, over multiple disease screens, the transgenic lines provided a level of resistance above that present in the nontransgenic control cultivar Bobwhite. In particular, we identified CM27, a line carrying a β-1,3-glucanase transgene that exhibited low FHB severity, low DON concentration, and low percent VSK in the field. Interestingly, in the field screens we observed lines, such as CM30, with significantly lower FHB severity and a high DON level. As seen in the greenhouse screens, these results are likely due to the variation in FHB disease screens. Variation in FHB readings from field grown plants can be difficult to control (Campbell and Lipps 1998).
Consistent with our results, Chen et al. (1999) and Anand et al. (2003) showed that overexpression of tlp-1 and a combination of β-1,3-glucanase and chitinase transgenes in wheat resulted in enhanced FHB disease in the greenhouse. Interestingly, these authors only detected enhanced resistance during early stages of disease progression. They interpreted the action of these transgenes as delaying the development of FHB. Unfortunately, field disease screens of their lines lacked resistance (Anand et al. 2003). For their field study, these authors used inoculated corn kernels, which provided a continuous source of inoculum, whereas in our field study, we sprayed fungal spores on the spikes twice. Thus, there is a distinct difference in the inoculation methodology between the two studies, which could lead to different disease reactions. Developmental differences such as the timing of flowering have resulted in different disease reactions to FHB. When inoculated grain is used for the inoculum, early heading plants can exhibit greater susceptibility than late heading plants as they are exposed to the inoculum for a longer period of time. In our study, we controlled the timing of inoculation through spraying the spikes, and we did not observe any obvious developmental differences in our transgenic lines. Thus, our results demonstrate that enhanced resistance to FHB can be obtained through overexpressing defense response genes.
To date, there are no wheat germplasm sources that exhibit immunity to FHB. The best available lines, such as Sumai 3 and Alsen, exhibit resistance to initial infection and spread of the disease but this resistance is partial and plants may become severely diseased when conditions are highly favorable for disease development. The transgenic lines described in this study may provide a potential wheat germplasm source for enhanced FHB resistance. Although the level of transgene resistance is not high enough to alone provide useful protection to FHB, our transgenic lines may extend and enhance FHB resistance germplasm when combined with other resistance sources. To increase the level of resistance, we crossed our β-1,3-glucanase and tlp-1 transgenic lines and combined the transgenes into a common background because developing lines with multiple transgenes in tobacco increased resistance to a fungal pathogen (Jach et al. 1995). We have also initiated crosses of our transgenic lines with the moderately resistant genotype, Alsen. Alsen contains the chromosome 3BS QTL for FHB resistance (Waldron et al. 1999). Our goal is to develop populations containing the 3BS QTL in combination with each of the three transgenes. Our expectation is that these combinations may result in enhanced resistance to FHB over the levels present in Alsen.
Acknowledgements
We are most grateful to Dr. Tom Clemente and Shirley Sato of the University of Nebraska and to Drs. Ann Blechl, and Pat Okubara USDA-ARS, Albany, CA for their advise and assistance in generating the transgenic wheat plants. We are also grateful to Drs. Blechl, Okubara and Kent McCue and for providing the pKM1 and pUBK BglII transformation constructs. We thank Dr. John Mundy of the Carlsberg Research Laboratory, Copenhagen, Denmark and Dr. David Collinge of the Department of Plant Pathology, Royal Veterinary and Agricultural University, Copenhagen, Denmark for providing barley β-1,3-glucanase and tlp-1 cDNAs, respectively. We also thank Dr. Peter Quail of the Plant Gene Expression Center, University of California at Berkeley for providing the pAHC25 and pAHC17 plasmids. We are indebted to Keith Hansen, Adam Dobberfuhl, Dong Tuong, Sarah Jutila, Alissa Cyrus, John Wiersma, Amar Elakkad, Karen Wennberg and Yanhong Dong for excellent technical assistance. Sanghyun Shin was supported by the Korea Research Foundation (KRF-2005-000-10035). This work was supported by grants from the U.S. Wheat and Barley Scab Initiative, Minnesota State Scab Initiative, and Minnesota Wheat Research and Promotion Council.
References
- Anand A, Zhou T, Trick HN, Gill BS, Bockus WW, Muthukrishnan S (2003) Greenhouse and field testing of transgenic wheat plants stably expressing genes for thaumatin-like protein, chitinase and glucanase against Fusarium graminearum. J Exp Bot 54:1101–1111 [DOI] [PubMed]
- Bai GH, Shaner G (1994) Scab of wheat: prospect for control. Plant Dis 78:760–766
- Bai GH, Shaner G (1996) Variation in Fusarium graminearum and cultivar resistance to wheat scab. Plant Dis 80:975–979
- Bernardo A, Bai G, Guo P, Xiao K, Guenzi AC, Ayoubi P (2006) Fusarium graminearum-induced changes in gene expression between head blight resistant and susceptible cultivars. Funct Integr Genomics, in press [DOI] [PubMed]
- Bieri S, Potrykus I, Futterer J (2003) Effects of combined expression of antifungal barley seed proteins in transgenic wheat on powdery mildew infection. Mol Breed 11:37–48 [DOI]
- Bliffeld M, Mundy J, Potrykus I, Futterer J (1999) Genetic engineering of wheat for increased resistance to powdery mildew disease. Theor Appl Genet 98:1079–1086 [DOI]
- Boddu J, Cho S, Kruger WM, Muehlbauer GJ (2006) Transcriptome analysis of the barley–Fusarium graminearum interaction. Mol Plant Microbe Interact 19:407–417 [DOI] [PubMed]
- Bohlmann H, Clausen S, Behnke S, Giese H, Hiller C, Reimann-Phillipp U, Schrader G, Barkholt V, Apel K (1988) Leaf-specific thionins of barley—a novel class of cell wall proteins toxic to plant-pathogenic fungi and possibly involved in the defense mechanism of plants. EMBO 7:1559–1565 [DOI] [PMC free article] [PubMed]
- Campbell KAG, Lipps PE (1998) Allocation of resources: sources of variation in Fusarium head blight screening nurseries. Phytopathology 88:1078–1086 [DOI] [PubMed]
- Chen WP, Chen PD, Liu DJ, Kynast R, Friebe B, Velazhahan R, Muthukrishnan S, Gill BS (1999) Development of wheat scab symptoms is delayed in transgenic wheat plants that constitutively express a rice thaumatin-like protein gene. Theor Appl Genet 99:755–760 [DOI]
- Christensen AH, Sharrock RA, Quail PH (1992) Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol Biol 18:675–689 [DOI] [PubMed]
- de la Peña RC, Murray TD, Jones SS (1996) Linkage relationships among eyespot resistance gene Pch2, endopeptidase Ep-A1b, and RFLP marker Xpsr121 on chromosome 7A of wheat. Plant Breed 115:273–275 [DOI]
- Epple P, Apel K, Bohlmann H (1997) Overexpression of an endogenous thionin enhances resistance of Arabidopsis against Fusarium oxysporum. Plant Cell 9:509–520 [DOI] [PMC free article] [PubMed]
- Evans CK, Xie W, Dill-Macky R, Mirocha CJ (2000) Biosynthesis of deoxynivalenol in spikelets of barley inoculated with macroconidia of Fusarium graminearum. Plant Dis 84:654–660 [DOI] [PubMed]
- Han FP, Fedak G, Ouellet T, Dan H, Somers DJ (2005) Mapping genes expressed in Fusarium graminearum-infected heads of wheat cultivar ‘Frontana’. Genome 48:88–96 [DOI] [PubMed]
- Jach G, Gornhardt B, Mundy J, Logemann J, Pinsdorf E, Leah R, Schell J, Maas C (1995) Enhanced quantitative resistance against fungal disease by combinatorial expression of different barley antifungal proteins in transgenic tobacco. Plant J 8:97–109 [DOI] [PubMed]
- Jones RK, Mirocha CJ (1999) Quality parameters in small grains from Minnesota affected by Fusarium head blight. Plant Dis 83:506–511 [DOI] [PubMed]
- Kang Z, Buchenauer H (2003) Immunocytochemical localizations of cell wall bound thionins and hydroxyproline-rich glycoproteins in Fusarium culmorum-infected wheat spikes. J Phytopathol 151:120–129 [DOI]
- Kang Z, Buchenauer H (2002) Immunocytochemical localization of β-1,3-glucanase and chitinase in Fusarium culmorum-infected wheat spikes. Physiol Mol Plant Pathol 60:141–153 [DOI]
- Kolb FL, Bai G-H, Muehlbauer GJ, Anderson JA, Smith KP, Fedak G (2001) Host plant resistance genes for Fusarium head blight: mapping and manipulation with molecular markers. Crop Sci 41:611–619
- Kong L, Anderson JM, Ohm HW (2005) Induction of wheat defense and stress-related genes in response to Fusarium graminearum. Genome 48:29–40 [DOI] [PubMed]
- Leah R, Tommerup H, Svendsen I, Mundy J (1991) Biochemical and molecular characterization of three barley seed proteins with antifungal properties. J Biol Chem 266:1564–1573 [PubMed]
- Leonard KJ, Bushnell WR (eds) (2003) Fusarium head blight of wheat and barley. American Phytopathological Society Press, St. Paul, MN
- Li WL, Faris JD, Muthukrishnan S, Liu DJ, Chen PD, Gill BS (2001) Isolation and characterization of novel cDNA clones of acidic chitinases and β-1,3-glucanases from wheat spikes infected by Fusarium graminearum. Theor Appl Genet 102:353–362 [DOI]
- Linthorst HJM (1991) Pathogenesis-related proteins of plants. Crit Rev Plant Sci 10:123–150
- Mackintosh CA, Garvin DF, Radmer LE, Heinen SJ, Muehlbauer GJ (2006) A model wheat cultivar for transformation to improve resistance to Fusarium Head Blight. Plant Cell Rep 25:313–319 [DOI] [PubMed]
- Makandar R, Essig JS, Schapaugh MA, Trick HN, Shah J (2006) Genetically engineered resistance to Fusarium head blight in wheat by expression of Arabidopsis NPR1. Mol Plant Microbe Interact 19:123–129 [DOI] [PubMed]
- McMullen M, Jones R, Gellenberg D (1997) Scab of wheat and barley: a re-emerging disease of devastating impact. Plant Dis 81:1340–1348 [DOI] [PubMed]
- Mirocha CJ, Kolaczkowski E, Xie W, Yu H, Jelen H (1998) Analysis of deoxynivalenol and its derivatives (batch and single kernel) using gas chromatography/mass spectrometry. J Agric Food Chem 46:1414–1418 [DOI]
- Mohammadi M, Kazemi H (2002) Changes in peroxidase and polyphenol oxidase activities in susceptible and resistant wheat heads inoculated with Fusarium graminearum and induced resistance. Plant Sci 162:491–498 [DOI]
- Muehlbauer GJ, Bushnell WR (2003) Transgenic approaches to resistance. In: Leonard KJ, Bushnell WR (eds) Fusarium head blight of wheat and barley. American Phytopathological Society Press, St. Paul, MN
- Nganje WE, Katiebie S, Wilson WW, Leistritz FL, Bangsund DA (2004) Economic impacts of Fusarium Head Blight in wheat and barley: 1993–2001. North Dakota State University Agribusiness and Applied Economics Report 538, p 53
- Oldach KH, Becker D, Lörz H (2001) Heterologous expression of genes mediating enhanced fungal resistance in transgenic wheat. Mol Plant Microbe Interact 14:832–838 [DOI] [PubMed]
- Pritsch C, Muehlbauer GJ, Bushnell WR, Somers DA, Vance CP (2000) Fungal development and induction of defense response genes during early infection of wheat spikes by Fusarium graminearum. Mol Plant Microbe Interact 13:159–169 [DOI] [PubMed]
- Pritsch C, Vance CP, Bushnell WR, Somers DA, Hohn TM, Muehlbauer GJ (2001) Systemic expression of defense response genes in wheat spikes as a response to Fusarium graminearum infection. Physiol Mol Plant Pathol 58:1–12 [DOI]
- Rudd JC, Horsley RD, McKendry AL, Elias EM (2001) Host plant resistance genes for Fusarium head blight: sources, mechanisms, and utility in conventional breeding systems. Crop Sci 41:620–627
- Sutton JC (1982) Epidemiology of wheat head blight and maize ear rot caused by Fusarium graminearum. Trans Br Mycol Soc 70:187–192
- Tuite J, Shaner G, Everson RJ (1990) Wheat scab in soft red winter wheat in Indiana in 1986 and its relation to quality measurements. Plant Dis 74:959–962
- Waldron BL, Moreno-Sevilla B, Anderson JA, Stack RW, Frohberg RC (1999) RFLP mapping of a QTL for Fusarium head blight resistance in wheat. Crop Sci 39:805–811
- Yun DJ, Ibeas JI, Lee H, Coca MA, Narasimhan ML, Uesono Y, Hasegawa PM, Pardo JM, Bressan RA (1998) Osmotin, a plant antifungal protein, subverts signal transduction to enhance fungal cell susceptibility. Mol Cell 1:807–812 [DOI] [PubMed]
- Zhou W, Kolb FL, Riechers DE (2005) Identification of proteins induced or upregulated by Fusarium head blight infection in the spikes of hexaploid wheat (Triticum aestivum). Genome 48:770–780 [DOI] [PubMed]


