Abstract
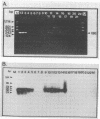
A rapid and simple method was developed to detect enteroviruses and hepatitis A virus (HAV) in sewage and ocean water. Sewage samples were concentrated by Centriprep-100 and Centricon-100 at 1,000 x g. Samples collected from estuary and near-shore surf zone ocean water in Southern California were concentrated by vortex flow filtration and microconcentration. Reverse transcriptase-polymerase chain reaction (RT-PCR), with enterovirus primers or HAV capsid-specific primers, was used to detect enteroviruses or HAV in all concentrated samples. A nonradioactive internal probe was used to confirm the amplified products. Results of seeding experiments indicated that at 4 degrees C, HAV was more persistent than poliovirus in seawater and both HAV and poliovirus persisted longer at 4 degrees C than at 25 degrees C. RT-PCR was at least 500-fold more sensitive than cell culture. Results were obtained within 5 h by RT-PCR, in contrast with the 5 days to 3 weeks required for cell culture.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atmar R. L., Metcalf T. G., Neill F. H., Estes M. K. Detection of enteric viruses in oysters by using the polymerase chain reaction. Appl Environ Microbiol. 1993 Feb;59(2):631–635. doi: 10.1128/aem.59.2.631-635.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leon R., Matsui S. M., Baric R. S., Herrmann J. E., Blacklow N. R., Greenberg H. B., Sobsey M. D. Detection of Norwalk virus in stool specimens by reverse transcriptase-polymerase chain reaction and nonradioactive oligoprobes. J Clin Microbiol. 1992 Dec;30(12):3151–3157. doi: 10.1128/jcm.30.12.3151-3157.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouvea V., Glass R. I., Woods P., Taniguchi K., Clark H. F., Forrester B., Fang Z. Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990 Feb;28(2):276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Estes M. K., Metcalf T. G. Detection of hepatitis A virus by hybridization with single-stranded RNA probes. Appl Environ Microbiol. 1987 Oct;53(10):2487–2495. doi: 10.1128/aem.53.10.2487-2495.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Wang J., Graham D. Y., Estes M. K. Detection of Norwalk virus in stool by polymerase chain reaction. J Clin Microbiol. 1992 Oct;30(10):2529–2534. doi: 10.1128/jcm.30.10.2529-2534.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecka H., Dubrou S., Prevot J., Marechal J., López-Pila J. M. Detection of naturally occurring enteroviruses in waters by reverse transcription, polymerase chain reaction, and hybridization. Appl Environ Microbiol. 1993 Apr;59(4):1213–1219. doi: 10.1128/aem.59.4.1213-1219.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon S. M., Binn L. N., Marchwicki R. H. Radioimmunofocus assay for quantitation of hepatitis A virus in cell cultures. J Clin Microbiol. 1983 May;17(5):834–839. doi: 10.1128/jcm.17.5.834-839.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis G. D., Metcalf T. G. Polyethylene glycol precipitation for recovery of pathogenic viruses, including hepatitis A virus and human rotavirus, from oyster, water, and sediment samples. Appl Environ Microbiol. 1988 Aug;54(8):1983–1988. doi: 10.1128/aem.54.8.1983-1988.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. H., Jiang S. C., Rose J. B. Concentration of viruses and dissolved DNA from aquatic environments by vortex flow filtration. Appl Environ Microbiol. 1991 Aug;57(8):2197–2204. doi: 10.1128/aem.57.8.2197-2204.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichard Scott L., Paul John H. Detection of Gene Expression in Genetically Engineered Microorganisms and Natural Phytoplankton Populations in the Marine Environment by mRNA Analysis. Appl Environ Microbiol. 1991 Jun;57(6):1721–1727. doi: 10.1128/aem.57.6.1721-1727.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotbart H. A. Enzymatic RNA amplification of the enteroviruses. J Clin Microbiol. 1990 Mar;28(3):438–442. doi: 10.1128/jcm.28.3.438-442.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan R. J., Goksøyr J., Bej A. K., Atlas R. M. Recovery of DNA from soils and sediments. Appl Environ Microbiol. 1988 Dec;54(12):2908–2915. doi: 10.1128/aem.54.12.2908-2915.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann J. Detection of rotavirus in sewage. Appl Environ Microbiol. 1981 Apr;41(4):1043–1045. doi: 10.1128/aem.41.4.1043-1045.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y. L., Olson B. H. Detection of low numbers of bacterial cells in soils and sediments by polymerase chain reaction. Appl Environ Microbiol. 1992 Feb;58(2):754–757. doi: 10.1128/aem.58.2.754-757.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y. L., Olson B. H. Rapid method for separation of bacterial DNA from humic substances in sediments for polymerase chain reaction. Appl Environ Microbiol. 1992 Jul;58(7):2292–2295. doi: 10.1128/aem.58.7.2292-2295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y. L., Palmer C. J., Sangermano L. R. Detection of Escherichia coli in sewage and sludge by polymerase chain reaction. Appl Environ Microbiol. 1993 Feb;59(2):353–357. doi: 10.1128/aem.59.2.353-357.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde J., Van R., Pickering L., Eiden J., Yolken R. Detection of rotaviruses in the day care environment by reverse transcriptase polymerase chain reaction. J Infect Dis. 1992 Sep;166(3):507–511. doi: 10.1093/infdis/166.3.507. [DOI] [PubMed] [Google Scholar]
- Zhou Y. J., Estes M. K., Jiang X., Metcalf T. G. Concentration and detection of hepatitis A virus and rotavirus from shellfish by hybridization tests. Appl Environ Microbiol. 1991 Oct;57(10):2963–2968. doi: 10.1128/aem.57.10.2963-2968.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]