Abstract
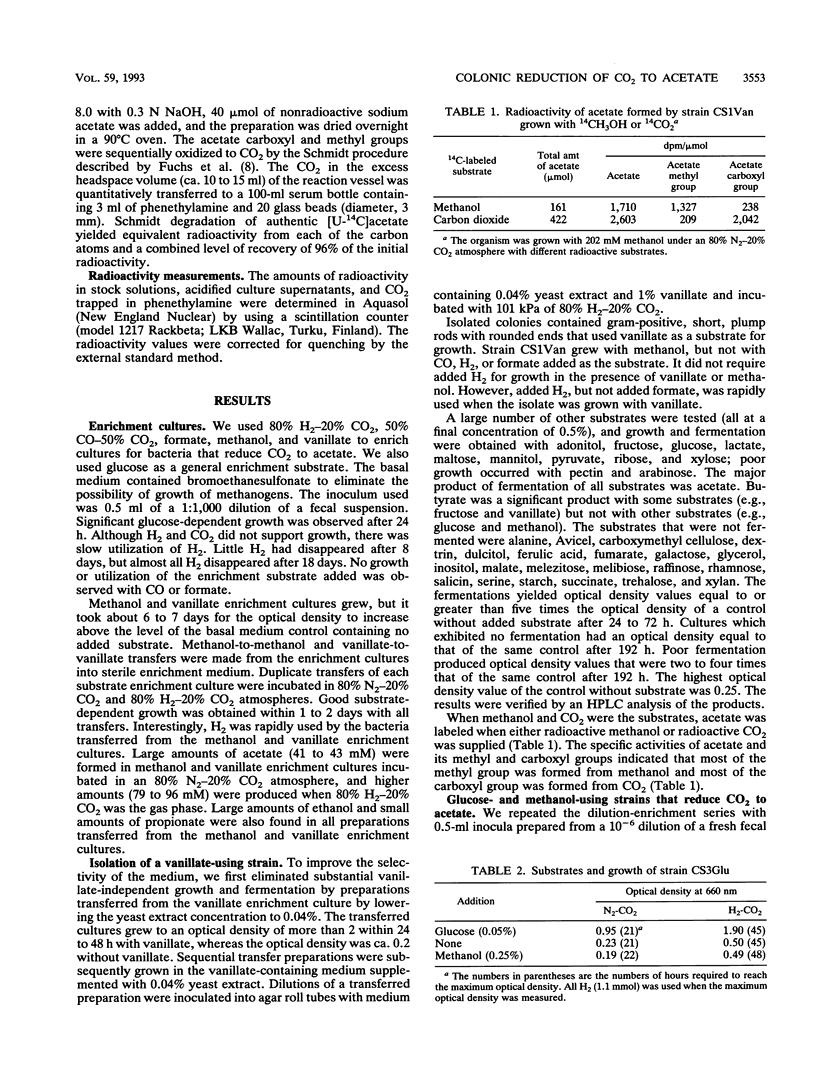
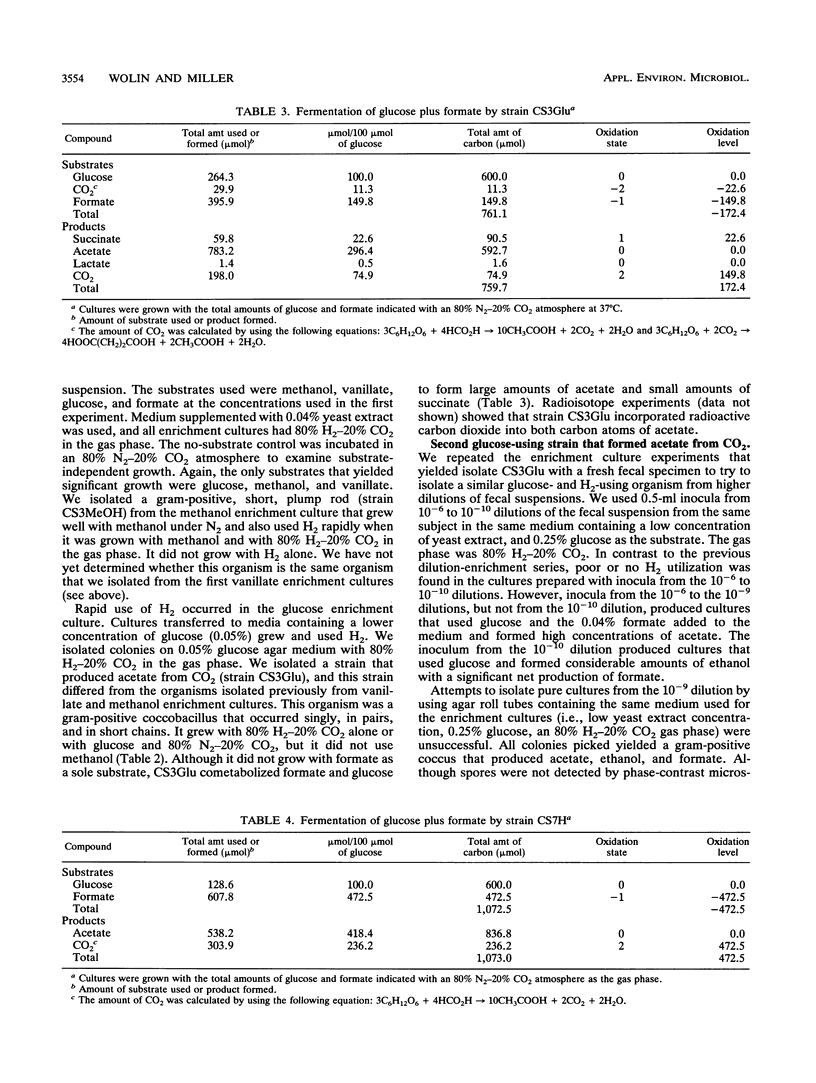
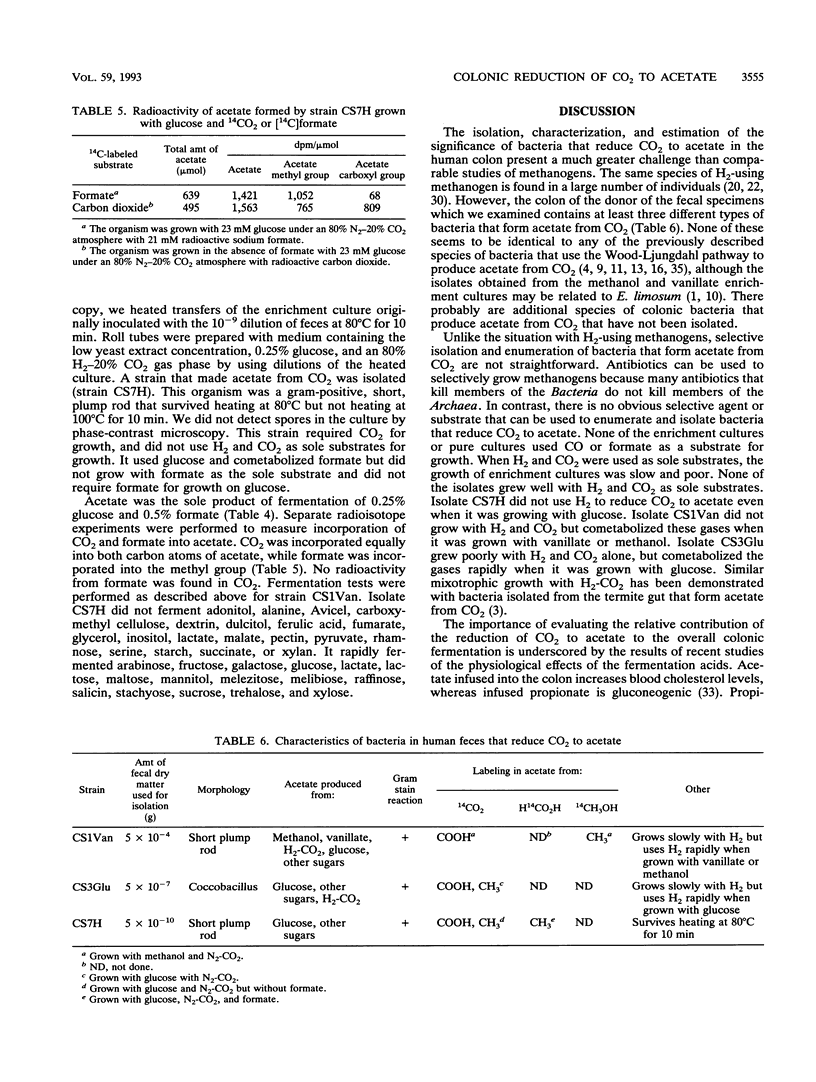
We used dilutions of fecal suspensions from a human volunteer to enrich cultures for bacteria that reduce CO2 to acetate in the colon. The soluble enrichment substrates used were glucose, methanol, formate, and vanillate, which were used with a gas phase that contained 80% N2 and 20% CO2. The gaseous enrichment substrates used were 80% H2-20% CO2 and 50% CO-50% CO2. We isolated three different strains that produced acetate from CO2. One strain produced acetate from methanol, vanillate, H2-CO2, glucose, and other sugars. The other two strains did not form acetate from methanol or vanillate. Both of the latter strains formed acetate from glucose and other sugars, but only one of these strains formed acetate from H2-CO2. Both of these strains cometabolized formate. However, none of the enrichment cultures or pure cultures used CO or formate as a substrate for growth. The two strains that produced acetate from H2 and CO2 grew slowly when the gases alone were used as substrates, but they rapidly cometabolized H2 and CO2 when they were grown with organic substrates. The ability of all of the strains to produce acetate from CO2 and/or other one-carbon precursors was verified by determining the radioactivity of the methyl and carboxyl groups of the acetate formed after growth with 14CO2 or other radioactively labeled one-carbon precursors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker H. A., Kamen M. D., Haas V. Carbon Dioxide Utilization in the Synthesis of Acetic and Butyric Acids by Butyribacterium Rettgeri. Proc Natl Acad Sci U S A. 1945 Nov;31(11):355–360. doi: 10.1073/pnas.31.11.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond J. H., Jr, Engel R. R., Levitt M. D. Factors influencing pulmonary methane excretion in man. An indirect method of studying the in situ metabolism of the methane-producing colonic bacteria. J Exp Med. 1971 Mar 1;133(3):572–588. doi: 10.1084/jem.133.3.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J. H., Pomare E. W., Branch W. J., Naylor C. P., Macfarlane G. T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987 Oct;28(10):1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich G. G., Goerlitz D. F., Bourell J. H., Eisen G. V., Godsy E. M. Liquid chromatographic procedure for fermentation product analysis in the identification of anaerobic bacteria. Appl Environ Microbiol. 1981 Nov;42(5):878–885. doi: 10.1128/aem.42.5.878-885.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genthner B. R., Davis C. L., Bryant M. P. Features of rumen and sewage sludge strains of Eubacterium limosum, a methanol- and H2-CO2-utilizing species. Appl Environ Microbiol. 1981 Jul;42(1):12–19. doi: 10.1128/aem.42.1.12-19.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greening R. C., Leedle J. A. Enrichment and isolation of Acetitomaculum ruminis, gen. nov., sp. nov.: acetogenic bacteria from the bovine rumen. Arch Microbiol. 1989;151(5):399–406. doi: 10.1007/BF00416597. [DOI] [PubMed] [Google Scholar]
- Kruh J. Effects of sodium butyrate, a new pharmacological agent, on cells in culture. Mol Cell Biochem. 1982 Feb 5;42(2):65–82. doi: 10.1007/BF00222695. [DOI] [PubMed] [Google Scholar]
- Lajoie S. F., Bank S., Miller T. L., Wolin M. J. Acetate production from hydrogen and [13C]carbon dioxide by the microflora of human feces. Appl Environ Microbiol. 1988 Nov;54(11):2723–2727. doi: 10.1128/aem.54.11.2723-2727.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt M. D. Intestinal gas production--recent advances in flatology. N Engl J Med. 1980 Jun 26;302(26):1474–1475. doi: 10.1056/NEJM198006263022610. [DOI] [PubMed] [Google Scholar]
- Ljungdahl L. G. The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu Rev Microbiol. 1986;40:415–450. doi: 10.1146/annurev.mi.40.100186.002215. [DOI] [PubMed] [Google Scholar]
- Lorowitz W. H., Bryant M. P. Peptostreptococcus productus strain that grows rapidly with CO as the energy source. Appl Environ Microbiol. 1984 May;47(5):961–964. doi: 10.1128/aem.47.5.961-964.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil N. I. The contribution of the large intestine to energy supplies in man. Am J Clin Nutr. 1984 Feb;39(2):338–342. doi: 10.1093/ajcn/39.2.338. [DOI] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl Microbiol. 1974 May;27(5):985–987. doi: 10.1128/am.27.5.985-987.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. Enumeration of Methanobrevibacter smithii in human feces. Arch Microbiol. 1982 Feb;131(1):14–18. doi: 10.1007/BF00451492. [DOI] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. Methanosphaera stadtmaniae gen. nov., sp. nov.: a species that forms methane by reducing methanol with hydrogen. Arch Microbiol. 1985 Mar;141(2):116–122. doi: 10.1007/BF00423270. [DOI] [PubMed] [Google Scholar]
- Pavlostathis S. G., Miller T. L., Wolin M. J. Fermentation of Insoluble Cellulose by Continuous Cultures of Ruminococcus albus. Appl Environ Microbiol. 1988 Nov;54(11):2655–2659. doi: 10.1128/aem.54.11.2655-2659.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger W. E. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology. 1982 Aug;83(2):424–429. [PubMed] [Google Scholar]
- Royall D., Wolever T. M., Jeejeebhoy K. N. Clinical significance of colonic fermentation. Am J Gastroenterol. 1990 Oct;85(10):1307–1312. [PubMed] [Google Scholar]
- Stephen A. M., Haddad A. C., Phillips S. F. Passage of carbohydrate into the colon. Direct measurements in humans. Gastroenterology. 1983 Sep;85(3):589–595. [PubMed] [Google Scholar]
- Tanaka Y., Bush K. K., Eguchi T., Ikekawa N., Taguchi T., Kobayashi Y., Higgins P. J. Effects of 1,25-dihydroxyvitamin D3 and its analogs on butyrate-induced differentiation of HT-29 human colonic carcinoma cells and on the reversal of the differentiated phenotype. Arch Biochem Biophys. 1990 Feb 1;276(2):415–423. doi: 10.1016/0003-9861(90)90740-p. [DOI] [PubMed] [Google Scholar]
- Thornton J. R., Dryden A., Kelleher J., Losowsky M. S. Super-efficient starch absorption. A risk factor for colonic neoplasia? Dig Dis Sci. 1987 Oct;32(10):1088–1091. doi: 10.1007/BF01300193. [DOI] [PubMed] [Google Scholar]
- Weaver G. A., Krause J. A., Miller T. L., Wolin M. J. Constancy of glucose and starch fermentations by two different human faecal microbial communities. Gut. 1989 Jan;30(1):19–25. doi: 10.1136/gut.30.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver G. A., Krause J. A., Miller T. L., Wolin M. J. Cornstarch fermentation by the colonic microbial community yields more butyrate than does cabbage fiber fermentation; cornstarch fermentation rates correlate negatively with methanogenesis. Am J Clin Nutr. 1992 Jan;55(1):70–77. doi: 10.1093/ajcn/55.1.70. [DOI] [PubMed] [Google Scholar]
- Weaver G. A., Krause J. A., Miller T. L., Wolin M. J. Incidence of methanogenic bacteria in a sigmoidoscopy population: an association of methanogenic bacteria and diverticulosis. Gut. 1986 Jun;27(6):698–704. doi: 10.1136/gut.27.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw A., Sleath K. Myth of the marsupial mother: home care of very low birth weight babies in Bogota, Colombia. Lancet. 1985 May 25;1(8439):1206–1208. doi: 10.1016/s0140-6736(85)92877-6. [DOI] [PubMed] [Google Scholar]
- Wolever T. M., Spadafora P., Eshuis H. Interaction between colonic acetate and propionate in humans. Am J Clin Nutr. 1991 Mar;53(3):681–687. doi: 10.1093/ajcn/53.3.681. [DOI] [PubMed] [Google Scholar]



