Abstract
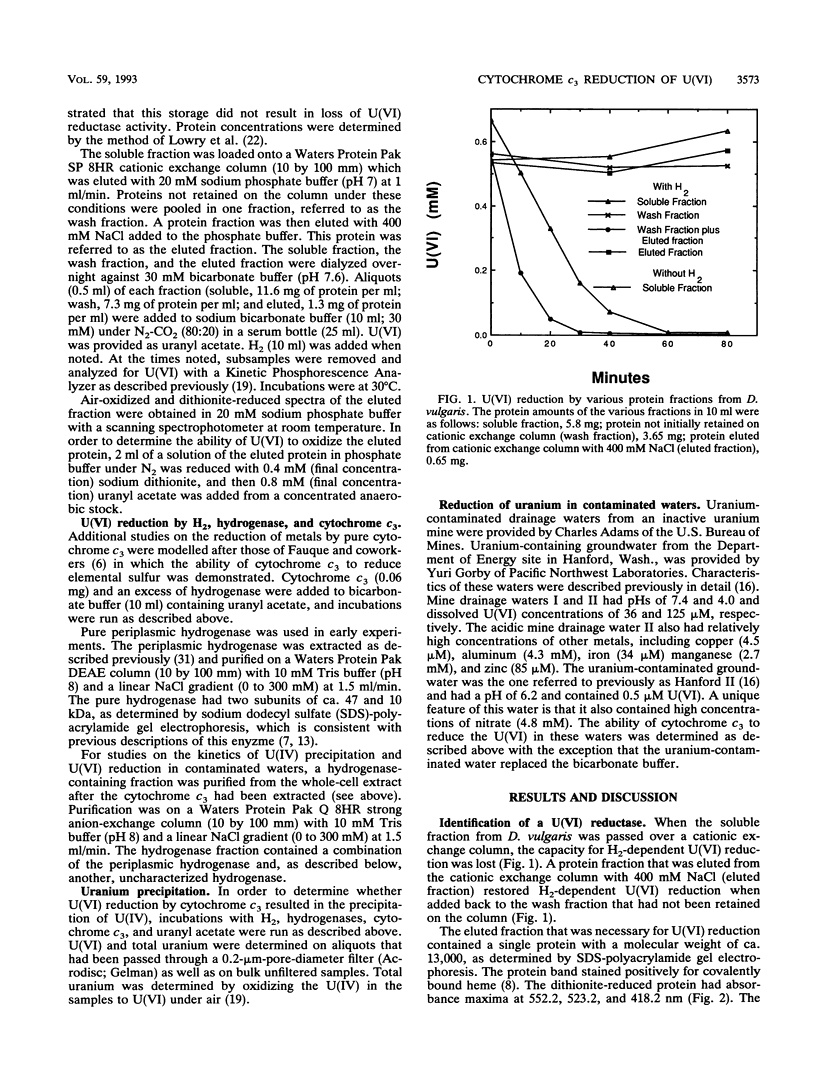
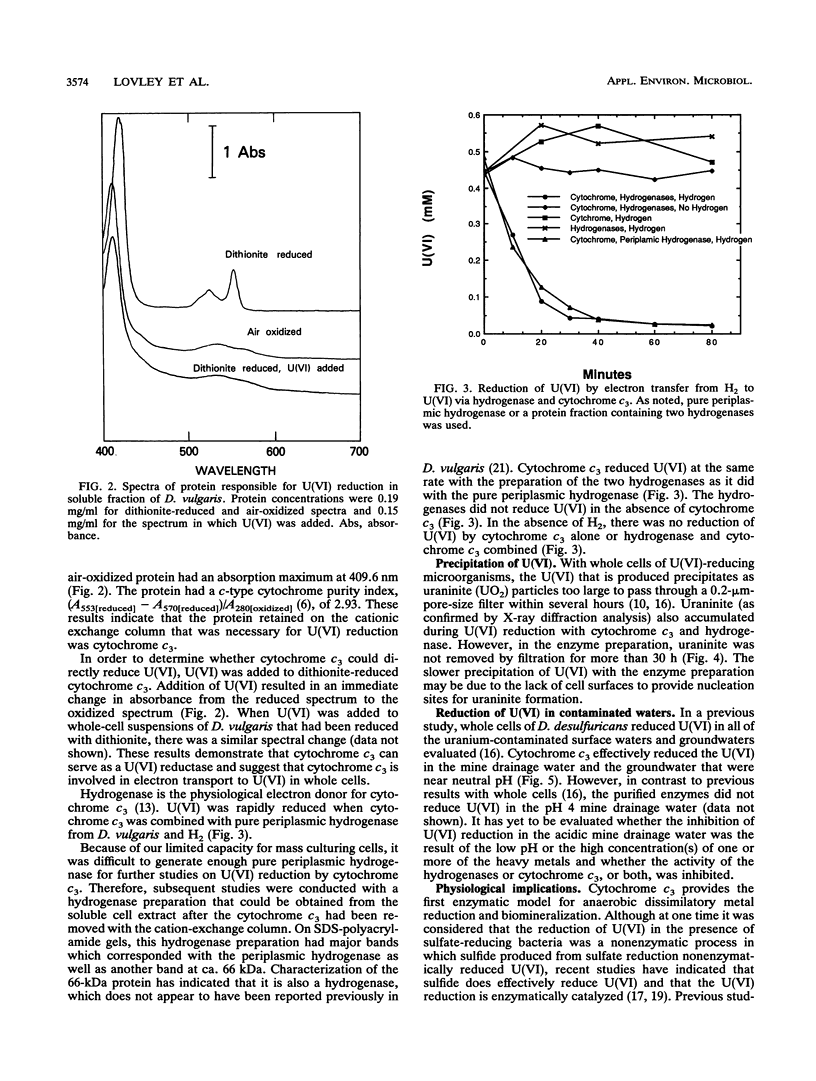
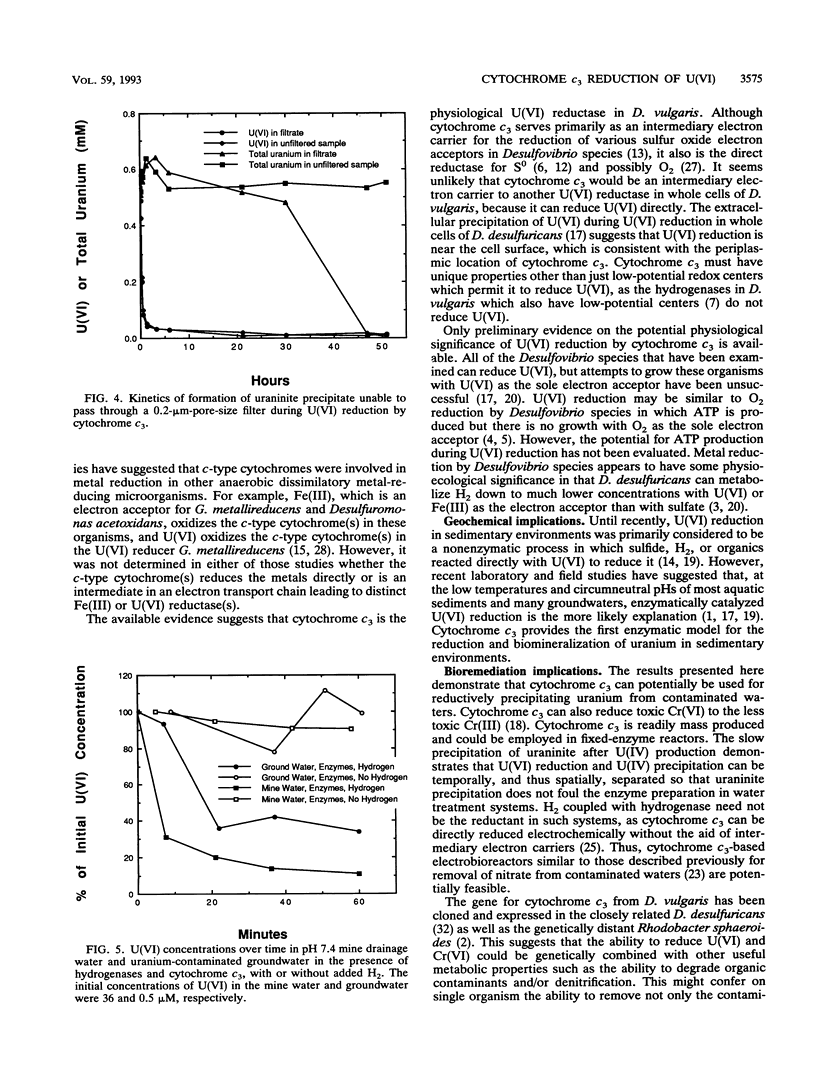
The mechanism for U(VI) reduction by Desulfovibrio vulgaris (Hildenborough) was investigated. The H2-dependent U(VI) reductase activity in the soluble fraction of the cells was lost when the soluble fraction was passed over a cationic exchange column which extracted cytochrome c3. Addition of cytochrome c3 back to the soluble fraction that had been passed over the cationic exchange column restored the U(VI)-reducing capacity. Reduced cytochrome c3 was oxidized by U(VI), as was a c-type cytochrome(s) in whole-cell suspensions. When cytochrome c3 was combined with hydrogenase, its physiological electron donor, U(VI) was reduced in the presence of H2. Hydrogenase alone could not reduce U(VI). Rapid U(VI) reduction was followed by a subsequent slow precipitation of the U(IV) mineral uraninite. Cytochrome c3 reduced U(VI) in a uranium-contaminated surface water and groundwater. Cytochrome c3 provides the first enzyme model for the reduction and biomineralization of uranium in sedimentary environments. Furthermore, the finding that cytochrome c3 can catalyze the reductive precipitation of uranium may aid in the development of fixed-enzyme reactors and/or organisms with enhanced U(VI)-reducing capacity for the bioremediation of uranium-contaminated waters and waste streams.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cannac V., Caffrey M. S., Voordouw G., Cusanovich M. A. Expression of the gene encoding cytochrome c3 from the sulfate-reducing bacterium Desulfovibrio vulgaris in the purple photosynthetic bacterium Rhodobacter sphaeroides. Arch Biochem Biophys. 1991 May 1;286(2):629–632. doi: 10.1016/0003-9861(91)90091-v. [DOI] [PubMed] [Google Scholar]
- Fauque G., Herve D., Le Gall J. Structure-function relationship in hemoproteins: the role of cytochrome c3 in the reduction of colloidal sulfur by sulfate-reducing bacteria. Arch Microbiol. 1979 Jun;121(3):261–264. doi: 10.1007/BF00425065. [DOI] [PubMed] [Google Scholar]
- Fauque G., Peck H. D., Jr, Moura J. J., Huynh B. H., Berlier Y., DerVartanian D. V., Teixeira M., Przybyla A. E., Lespinat P. A., Moura I. The three classes of hydrogenases from sulfate-reducing bacteria of the genus Desulfovibrio. FEMS Microbiol Rev. 1988 Dec;4(4):299–344. doi: 10.1111/j.1574-6968.1988.tb02748.x. [DOI] [PubMed] [Google Scholar]
- Francis R. T., Jr, Becker R. R. Specific indication of hemoproteins in polyacrylamide gels using a double-staining process. Anal Biochem. 1984 Feb;136(2):509–514. doi: 10.1016/0003-2697(84)90253-7. [DOI] [PubMed] [Google Scholar]
- Gaines C. G., Lodge J. S., Arceneaux J. E., Byers B. R. Ferrisiderophore reductase activity associated with an aromatic biosynthetic enzyme complex in Bacillus subtilis. J Bacteriol. 1981 Nov;148(2):527–533. doi: 10.1128/jb.148.2.527-533.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyer M., Page W. J. Ferric reductase activity in Azotobacter vinelandii and its inhibition by Zn2+. J Bacteriol. 1989 Jul;171(7):4031–4037. doi: 10.1128/jb.171.7.4031-4037.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Le Faou A., Rajagopal B. S., Daniels L., Fauque G. Thiosulfate, polythionates and elemental sulfur assimilation and reduction in the bacterial world. FEMS Microbiol Rev. 1990 Aug;6(4):351–381. doi: 10.1111/j.1574-6968.1990.tb04107.x. [DOI] [PubMed] [Google Scholar]
- Lovley D. R. Dissimilatory metal reduction. Annu Rev Microbiol. 1993;47:263–290. doi: 10.1146/annurev.mi.47.100193.001403. [DOI] [PubMed] [Google Scholar]
- Lovley D. R., Giovannoni S. J., White D. C., Champine J. E., Phillips E. J., Gorby Y. A., Goodwin S. Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch Microbiol. 1993;159(4):336–344. doi: 10.1007/BF00290916. [DOI] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Reduction of uranium by Desulfovibrio desulfuricans. Appl Environ Microbiol. 1992 Mar;58(3):850–856. doi: 10.1128/aem.58.3.850-856.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody M. D., Dailey H. A. Ferric iron reductase of Rhodopseudomonas sphaeroides. J Bacteriol. 1985 Sep;163(3):1120–1125. doi: 10.1128/jb.163.3.1120-1125.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden E. E., Lovley D. R. Dissimilatory Fe(III) Reduction by the Marine Microorganism Desulfuromonas acetoxidans. Appl Environ Microbiol. 1993 Mar;59(3):734–742. doi: 10.1128/aem.59.3.734-742.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiering N., Kabsch W., Moore M. J., Distefano M. D., Walsh C. T., Pai E. F. Structure of the detoxification catalyst mercuric ion reductase from Bacillus sp. strain RC607. Nature. 1991 Jul 11;352(6331):168–172. doi: 10.1038/352168a0. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Miyata N., Horitsu H., Kawai K., Takamizawa K., Tai Y., Okazaki M. NAD(P)H-dependent chromium (VI) reductase of Pseudomonas ambigua G-1: a Cr(V) intermediate is formed during the reduction of Cr(VI) to Cr(III). J Bacteriol. 1992 Aug;174(16):5340–5345. doi: 10.1128/jb.174.16.5340-5345.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voordouw G., Pollock W. B., Bruschi M., Guerlesquin F., Rapp-Giles B. J., Wall J. D. Functional expression of Desulfovibrio vulgaris Hildenborough cytochrome c3 in Desulfovibrio desulfuricans G200 after conjugational gene transfer from Escherichia coli. J Bacteriol. 1990 Oct;172(10):6122–6126. doi: 10.1128/jb.172.10.6122-6126.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Westen H. M., Mayhew S. G., Veeger C. Separation of hydrogenase from intact cells of Desulfovibrio vulgaris. Purification and properties. FEBS Lett. 1978 Feb 1;86(1):122–126. doi: 10.1016/0014-5793(78)80112-4. [DOI] [PubMed] [Google Scholar]



