Abstract
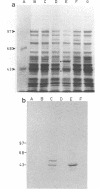
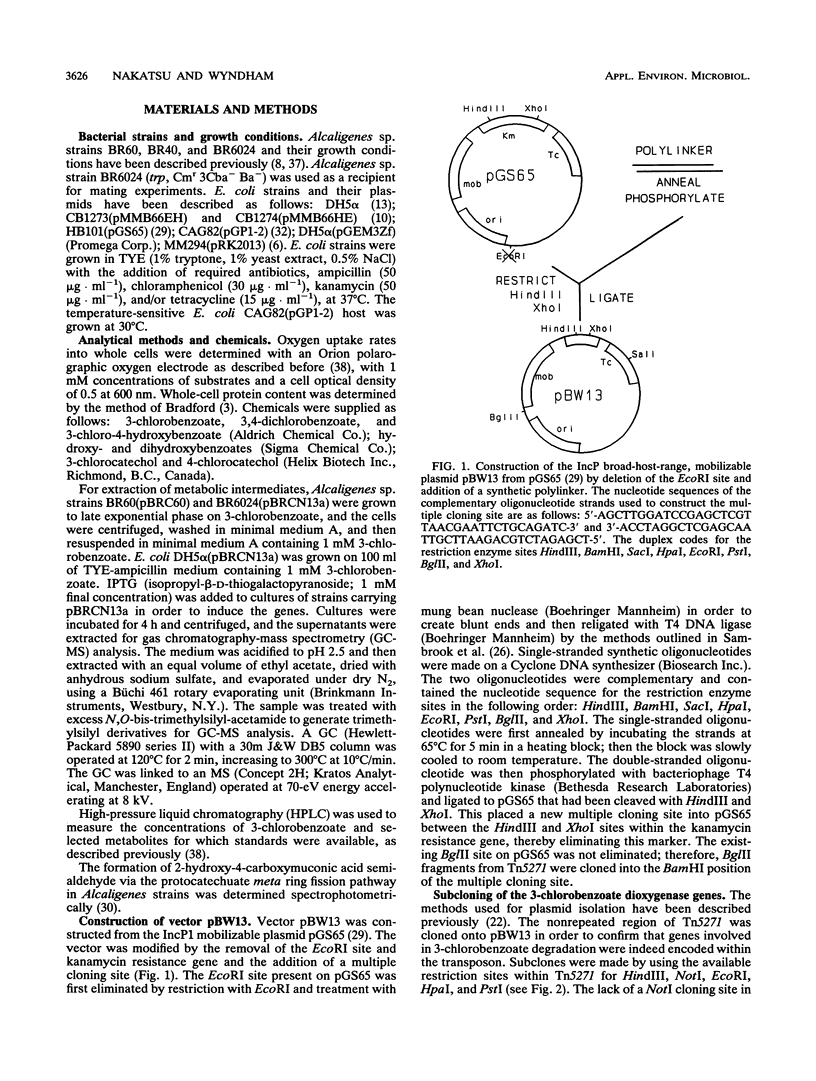
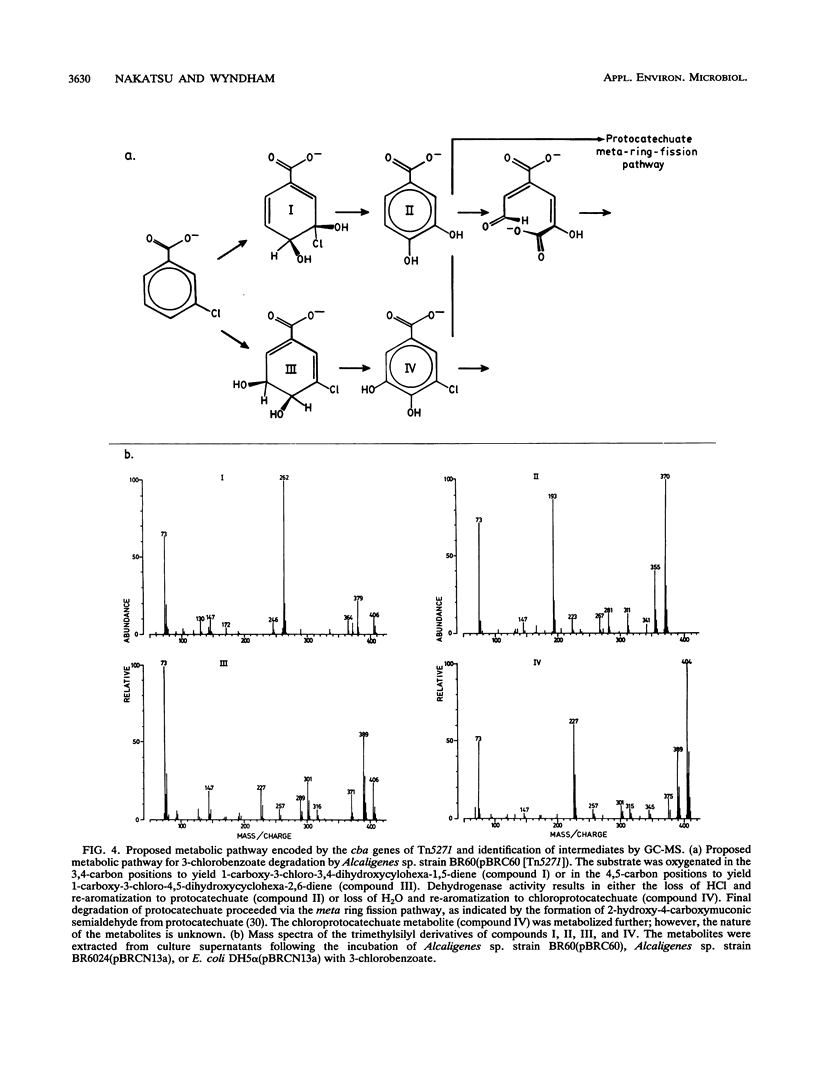
Growth on 3-chlorobenzoate was found to induce the enzymes of the protocatechuate meta ring fission pathway in Alcaligenes sp. strain BR60. The chlorobenzoate catabolic genes, designated cba, were localized to a 3.7-kb NotI-EcoRI fragment within the nonrepeated region of the composite transposon Tn5271. The cba genes were cloned onto two broad-host-range vectors and expressed in Escherichia coli and Alcaligenes sp. strain BR6024. In E. coli, expression of the cba genes with the IPTG (isopropyl-beta-D-thiogalactopyranoside)-inducible tac promoter of the IncQ vector pMMB66HE resulted in the production of protocatechuate and chlorodihydroxybenzoate metabolites of 3-chlorobenzoate. Expression of this construct in one orientation resulted in the formation of two polypeptides 51 and 42 kDa in size. This result was confirmed by subcloning into pGEM3Zf and then incorporating L-35S-methionine into newly synthesized proteins, using the thermally regulated T7 polymerase-promoter system. Introduction of the NotI-EcoRI fragment into Alcaligenes sp. strain BR6024 (Cba-P), using the IncP broad-host-range, mobilizable plasmid pBW13, restored the 3-chlorobenzoate-degradative phenotype and resulted in the accumulation of protocatechuate and chlorodihydroxybenzoate intermediates. The data indicate that a two-component dioxygenase specified by Tn5271 oxidizes 3-chlorobenzoate at the 3,4- or 4,5-positions. This activity extends the range of pathways for chloroaromatic compounds known to be functional in the environment. The new pathway avoids the toxicity attributed to the accumulation of chlorocatechol metabolites in bacteria degrading chlorobenzoates.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Engesser K. H., Schmidt E., Knackmuss H. J. Adaptation of Alcaligenes eutrophus B9 and Pseudomonas sp. B13 to 2-Fluorobenzoate as Growth Substrate. Appl Environ Microbiol. 1980 Jan;39(1):68–73. doi: 10.1128/aem.39.1.68-73.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetzner S., Müller R., Lingens F. Purification and some properties of 2-halobenzoate 1,2-dioxygenase, a two-component enzyme system from Pseudomonas cepacia 2CBS. J Bacteriol. 1992 Jan;174(1):279–290. doi: 10.1128/jb.174.1.279-290.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz B., Chakrabarty A. M. Organization and nucleotide sequence determination of a gene cluster involved in 3-chlorocatechol degradation. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4460–4464. doi: 10.1073/pnas.84.13.4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulthorpe R. R., Wyndham R. C. Involvement of a chlorobenzoate-catabolic transposon, Tn5271, in community adaptation to chlorobiphenyl, chloroaniline, and 2,4-dichlorophenoxyacetic acid in a freshwater ecosystem. Appl Environ Microbiol. 1992 Jan;58(1):314–325. doi: 10.1128/aem.58.1.314-325.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulthorpe R. R., Wyndham R. C. Transfer and Expression of the Catabolic Plasmid pBRC60 in Wild Bacterial Recipients in a Freshwater Ecosystem. Appl Environ Microbiol. 1991 May;57(5):1546–1553. doi: 10.1128/aem.57.5.1546-1553.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürste J. P., Pansegrau W., Frank R., Blöcker H., Scholz P., Bagdasarian M., Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48(1):119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Hensley M., Yoshioka H., Mabry T. J. Formation of (+)-cis-2,3-dihydroxy-1-methylcyclohexa-4,6-diene from toluene by Pseudomonas putida. Biochemistry. 1970 Mar 31;9(7):1626–1630. doi: 10.1021/bi00809a023. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hickey W. J., Focht D. D. Degradation of mono-, di-, and trihalogenated benzoic acids by Pseudomonas aeruginosa JB2. Appl Environ Microbiol. 1990 Dec;56(12):3842–3850. doi: 10.1128/aem.56.12.3842-3850.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holben W. E., Schroeter B. M., Calabrese V. G., Olsen R. H., Kukor J. K., Biederbeck V. O., Smith A. E., Tiedje J. M. Gene probe analysis of soil microbial populations selected by amendment with 2,4-dichlorophenoxyacetic acid. Appl Environ Microbiol. 1992 Dec;58(12):3941–3948. doi: 10.1128/aem.58.12.3941-3948.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten P. J., Chapman P. J., Dagley S. Enzymatic release of halogens or methanol from some substituted protocatechuic acids. J Bacteriol. 1985 May;162(2):693–697. doi: 10.1128/jb.162.2.693-697.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehrbach P. R., Zeyer J., Reineke W., Knackmuss H. J., Timmis K. N. Enzyme recruitment in vitro: use of cloned genes to extend the range of haloaromatics degraded by Pseudomonas sp. strain B13. J Bacteriol. 1984 Jun;158(3):1025–1032. doi: 10.1128/jb.158.3.1025-1032.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locher H. H., Leisinger T., Cook A. M. 4-Sulphobenzoate 3,4-dioxygenase. Purification and properties of a desulphonative two-component enzyme system from Comamonas testosteroni T-2. Biochem J. 1991 Mar 15;274(Pt 3):833–842. doi: 10.1042/bj2740833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus A., Krekel D., Lingens F. Purification and some properties of component A of the 4-chlorophenylacetate 3,4-dioxygenase from Pseudomonas species strain CBS. J Biol Chem. 1986 Sep 25;261(27):12883–12888. [PubMed] [Google Scholar]
- Mires M. H., Alexander C. H. The prophylactic treatment tuberculosis. Del Med J. 1972 Jul;44(7):187–190. [PubMed] [Google Scholar]
- Nakatsu C., Ng J., Singh R., Straus N., Wyndham C. Chlorobenzoate catabolic transposon Tn5271 is a composite class I element with flanking class II insertion sequences. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8312–8316. doi: 10.1073/pnas.88.19.8312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidle E. L., Shapiro M. K., Ornston L. N. Cloning and expression in Escherichia coli of Acinetobacter calcoaceticus genes for benzoate degradation. J Bacteriol. 1987 Dec;169(12):5496–5503. doi: 10.1128/jb.169.12.5496-5503.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidle E., Hartnett C., Ornston L. N., Bairoch A., Rekik M., Harayama S. cis-diol dehydrogenases encoded by the TOL pWW0 plasmid xylL gene and the Acinetobacter calcoaceticus chromosomal benD gene are members of the short-chain alcohol dehydrogenase superfamily. Eur J Biochem. 1992 Feb 15;204(1):113–120. doi: 10.1111/j.1432-1033.1992.tb16612.x. [DOI] [PubMed] [Google Scholar]
- Reineke W., Knackmuss H. J. Microbial degradation of haloaromatics. Annu Rev Microbiol. 1988;42:263–287. doi: 10.1146/annurev.mi.42.100188.001403. [DOI] [PubMed] [Google Scholar]
- Scholten J. D., Chang K. H., Babbitt P. C., Charest H., Sylvestre M., Dunaway-Mariano D. Novel enzymic hydrolytic dehalogenation of a chlorinated aromatic. Science. 1991 Jul 12;253(5016):182–185. doi: 10.1126/science.1853203. [DOI] [PubMed] [Google Scholar]
- Selvaraj G., Iyer V. N. A small mobilizable IncP group plasmid vector packageable into bacteriophage lambda capsids in vitro. Plasmid. 1985 Jan;13(1):70–74. doi: 10.1016/0147-619x(85)90057-5. [DOI] [PubMed] [Google Scholar]
- Sondossi M., Sylvestre M., Ahmad D. Effects of chlorobenzoate transformation on the Pseudomonas testosteroni biphenyl and chlorobiphenyl degradation pathway. Appl Environ Microbiol. 1992 Feb;58(2):485–495. doi: 10.1128/aem.58.2.485-495.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvestre M., Mailhiot K., Ahmad D., Massé R. Isolation and preliminary characterization of a 2-chlorobenzoate degrading Pseudomonas. Can J Microbiol. 1989 Apr;35(4):439–443. doi: 10.1139/m89-067. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walia S., Khan A., Rosenthal N. Construction and applications of DNA probes for detection of polychlorinated biphenyl-degrading genotypes in toxic organic-contaminated soil environments. Appl Environ Microbiol. 1990 Jan;56(1):254–259. doi: 10.1128/aem.56.1.254-259.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelis M. L., Palleroni N. J., Stanier R. Y. The metabolism of aromatic acids by Pseudomonas testosteroni and P. acidovorans. Arch Mikrobiol. 1967;59(1):302–314. doi: 10.1007/BF00406344. [DOI] [PubMed] [Google Scholar]
- Wyndham R. C. Evolved aniline catabolism in Acinetobacter calcoaceticus during continuous culture of river water. Appl Environ Microbiol. 1986 Apr;51(4):781–789. doi: 10.1128/aem.51.4.781-789.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyndham R. C., Singh R. K., Straus N. A. Catabolic instability, plasmid gene deletion and recombination in Alcaligenes sp. BR60. Arch Microbiol. 1988;150(3):237–243. doi: 10.1007/BF00407786. [DOI] [PubMed] [Google Scholar]
- Wyndham R. C., Straus N. A. Chlorobenzoate catabolism and interactions between Alcaligenes and Pseudomonas species from Bloody Run Creek. Arch Microbiol. 1988;150(3):230–236. doi: 10.1007/BF00407785. [DOI] [PubMed] [Google Scholar]