Abstract
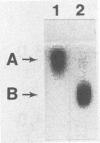
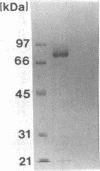
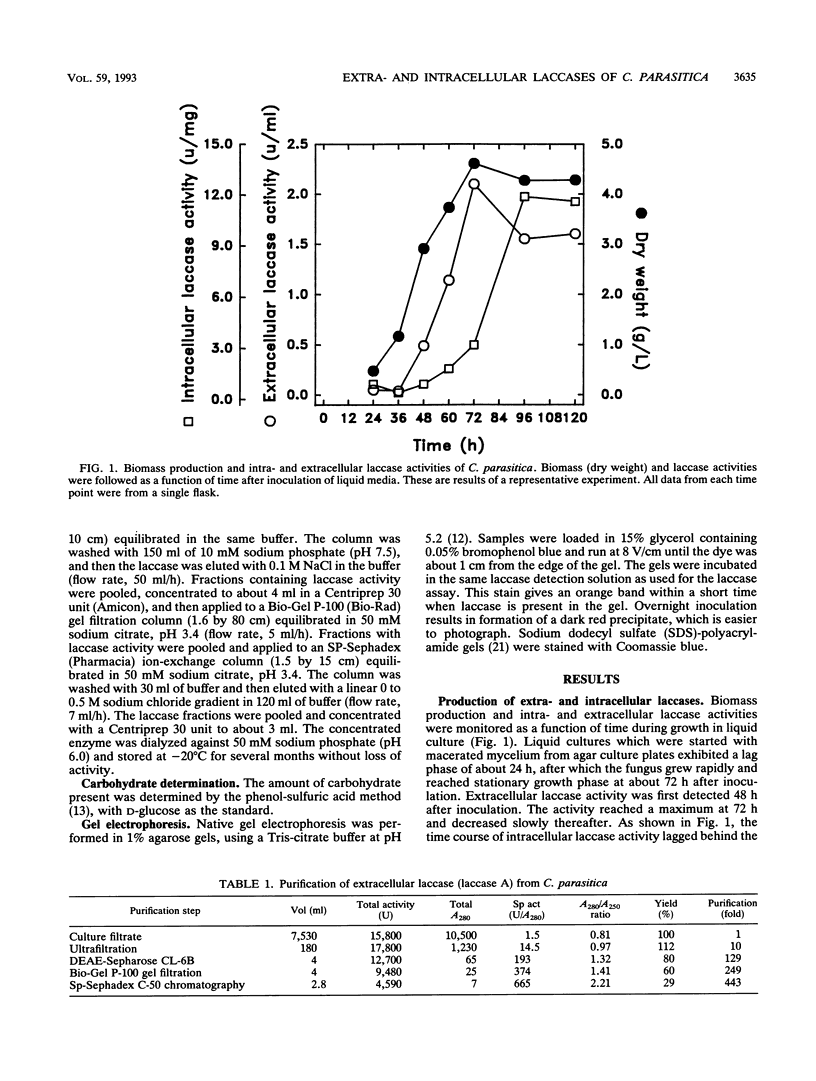
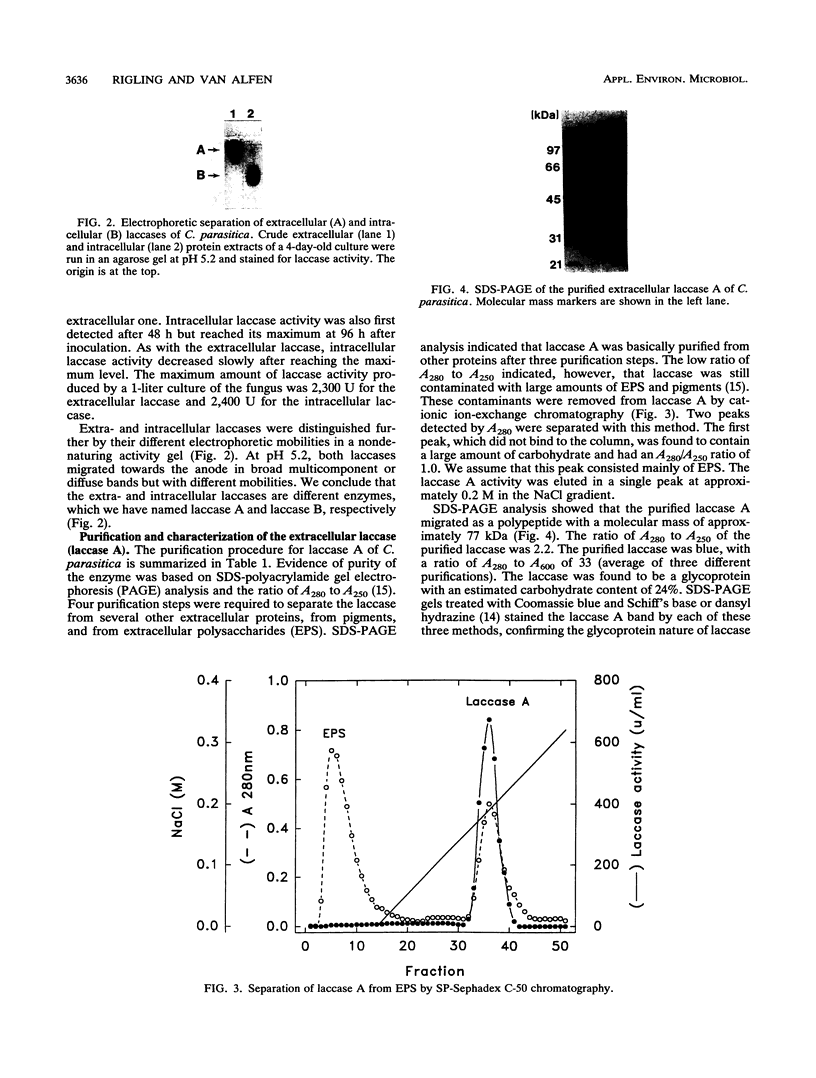
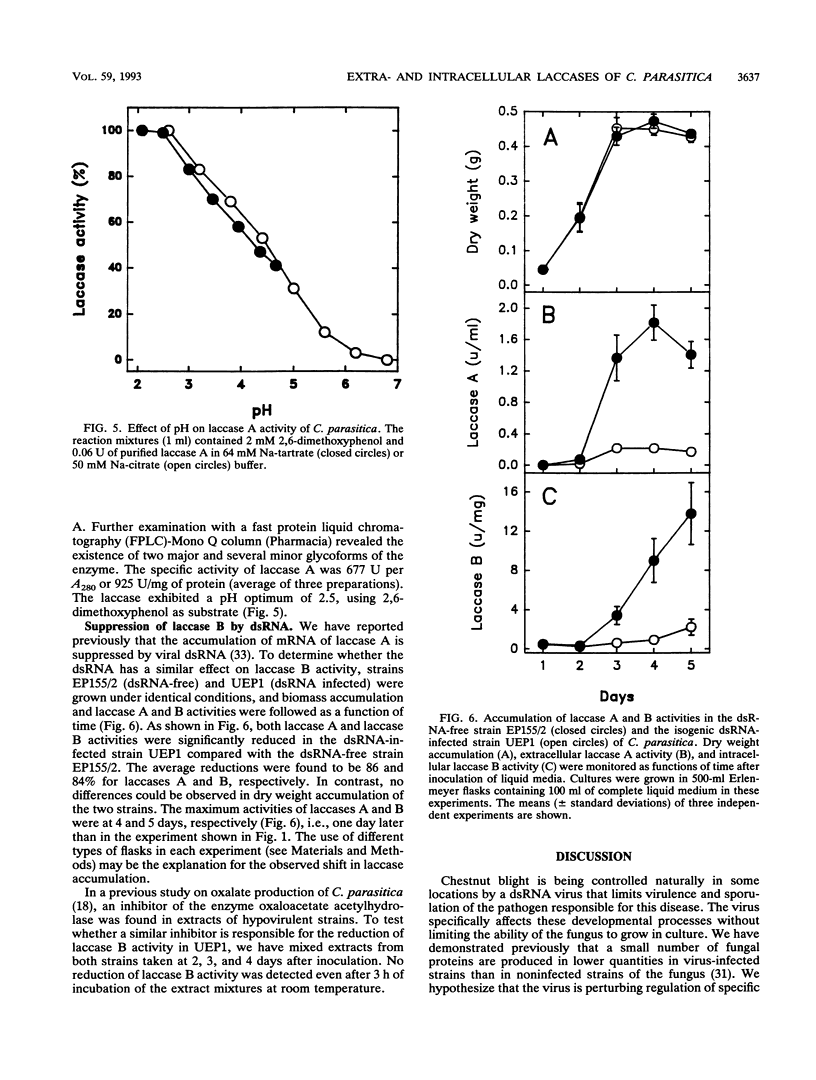
A double-stranded RNA virus of the chestnut blight pathogen, Cryphonectria parasitica, has been shown previously to reduce accumulation of mRNAs of extracellular laccase (laccase A) produced by this fungus. Both extra- and intracellular laccases have been detected after growth of the fungus in liquid culture. In addition to cellular localization, the two laccases are distinguishable by time of appearance during growth and electrophoretic mobility. Laccase A was purified from the culture filtrate by standard protein purification procedures. The enzyme was characterized as a glycoprotein with a molecular mass of approximately 77 kDa. Both laccase A and laccase B activities were significantly reduced in the hypovirulent (double-stranded RNA-infected) strain UEP1 compared with the isogenic virulent (double-stranded RNA-free) strain EP155/2.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bollag J. M., Leonowicz A. Comparative studies of extracellular fungal laccases. Appl Environ Microbiol. 1984 Oct;48(4):849–854. doi: 10.1128/aem.48.4.849-854.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollag J. M., Shuttleworth K. L., Anderson D. H. Laccase-mediated detoxification of phenolic compounds. Appl Environ Microbiol. 1988 Dec;54(12):3086–3091. doi: 10.1128/aem.54.12.3086-3091.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carpenter C. E., Mueller R. J., Kazmierczak P., Zhang L., Villalon D. K., Van Alfen N. K. Effect of a virus on accumulation of a tissue-specific cell-surface protein of the fungus Cryphonectria (Endothia) parasitica. Mol Plant Microbe Interact. 1992 Jan-Feb;5(1):55–61. doi: 10.1094/mpmi-5-055. [DOI] [PubMed] [Google Scholar]
- Choi G. H., Larson T. G., Nuss D. L. Molecular analysis of the laccase gene from the chestnut blight fungus and selective suppression of its expression in an isogenic hypovirulent strain. Mol Plant Microbe Interact. 1992 Mar-Apr;5(2):119–128. doi: 10.1094/mpmi-5-119. [DOI] [PubMed] [Google Scholar]
- Clutterbuck A. J. Absence of laccase from yellow-spored mutants of Aspergillus nidulans. J Gen Microbiol. 1972 May;70(3):423–435. doi: 10.1099/00221287-70-3-423. [DOI] [PubMed] [Google Scholar]
- Eckhardt A. E., Hayes C. E., Goldstein I. J. A sensitive fluorescent method for the detection of glycoproteins in polyacrylamide gels. Anal Biochem. 1976 May 21;73(1):192–197. doi: 10.1016/0003-2697(76)90154-8. [DOI] [PubMed] [Google Scholar]
- Froehner S. C., Eriksson K. E. Purification and properties of Neurospora crassa laccase. J Bacteriol. 1974 Oct;120(1):458–465. doi: 10.1128/jb.120.1.458-465.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germann U. A., Müller G., Hunziker P. E., Lerch K. Characterization of two allelic forms of Neurospora crassa laccase. Amino- and carboxyl-terminal processing of a precursor. J Biol Chem. 1988 Jan 15;263(2):885–896. [PubMed] [Google Scholar]
- Karhunen E., Niku-Paavola M. L., Viikari L., Haltia T., van der Meer R. A., Duine J. A. A novel combination of prosthetic groups in a fungal laccase; PQQ and two copper atoms. FEBS Lett. 1990 Jul 2;267(1):6–8. doi: 10.1016/0014-5793(90)80273-l. [DOI] [PubMed] [Google Scholar]
- Kersten P. J., Kalyanaraman B., Hammel K. E., Reinhammar B., Kirk T. K. Comparison of lignin peroxidase, horseradish peroxidase and laccase in the oxidation of methoxybenzenes. Biochem J. 1990 Jun 1;268(2):475–480. doi: 10.1042/bj2680475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larson T. G., Choi G. H., Nuss D. L. Regulatory pathways governing modulation of fungal gene expression by a virulence-attenuating mycovirus. EMBO J. 1992 Dec;11(12):4539–4548. doi: 10.1002/j.1460-2075.1992.tb05555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch K., Deinum J., Reinhammar B. The state of copper in Neurospora laccase. Biochim Biophys Acta. 1978 May 24;534(1):7–14. doi: 10.1016/0005-2795(78)90470-1. [DOI] [PubMed] [Google Scholar]
- Linden R. M., Schilling B. C., Germann U. A., Lerch K. Regulation of laccase synthesis in induced Neurospora crassa cultures. Curr Genet. 1991 May;19(5):375–381. doi: 10.1007/BF00309598. [DOI] [PubMed] [Google Scholar]
- Molitoris H. P., Esser K. Die Phenoloxydasen des Ascomyceten Podospora anserina. V. Eigenschaften der Laccase I nach weiterer Reinigung. Arch Mikrobiol. 1970;72(3):267–296. [PubMed] [Google Scholar]
- Powell W. A., Jr, Van Alfen N. K. Two nonhomologus viruses of Cryphonectria (Endothia) parasitica reduce accumulation of specific virulence-associated polypeptides. J Bacteriol. 1987 Nov;169(11):5324–5326. doi: 10.1128/jb.169.11.5324-5326.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell W. A., Van Alfen N. K. Differential accumulation of poly(A)+ RNA between virulent and double-stranded RNA-induced hypovirulent strains of Cryphonectria (Endothia) parasitica. Mol Cell Biol. 1987 Oct;7(10):3688–3693. doi: 10.1128/mcb.7.10.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigling D., Van Alfen N. K. Regulation of laccase biosynthesis in the plant-pathogenic fungus Cryphonectria parasitica by double-stranded RNA. J Bacteriol. 1991 Dec;173(24):8000–8003. doi: 10.1128/jb.173.24.8000-8003.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]