Abstract
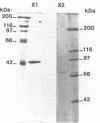
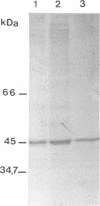
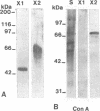
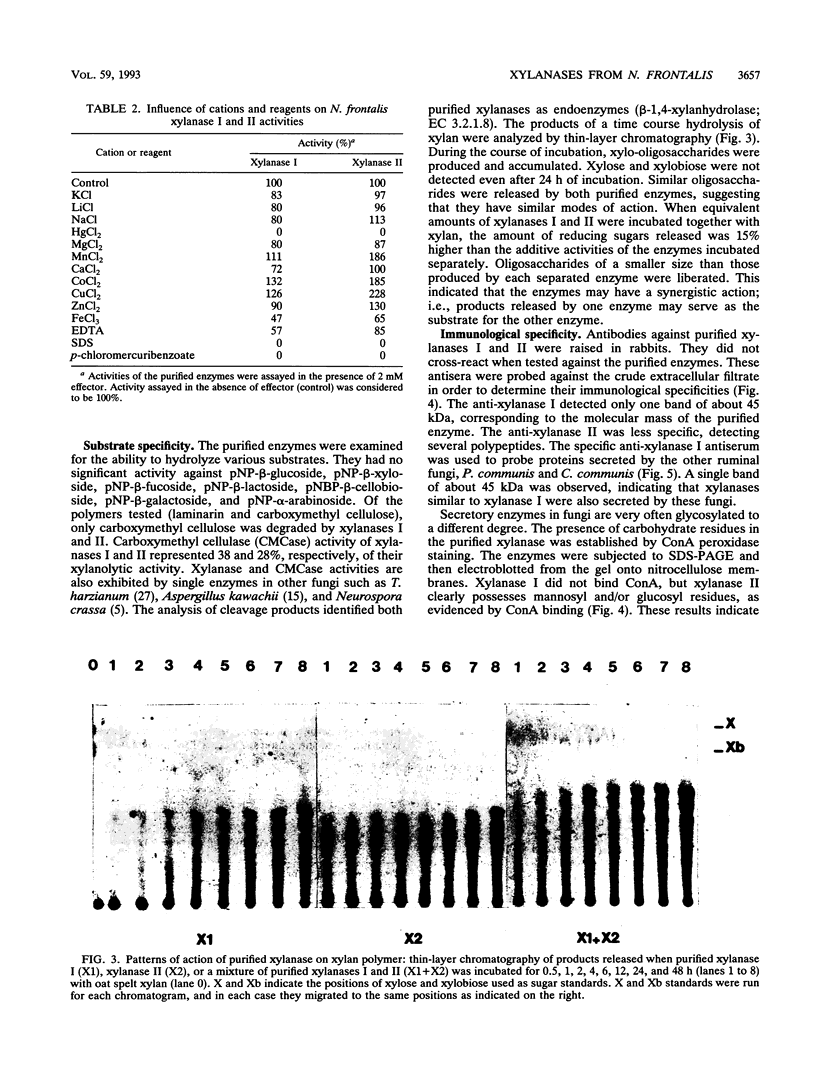
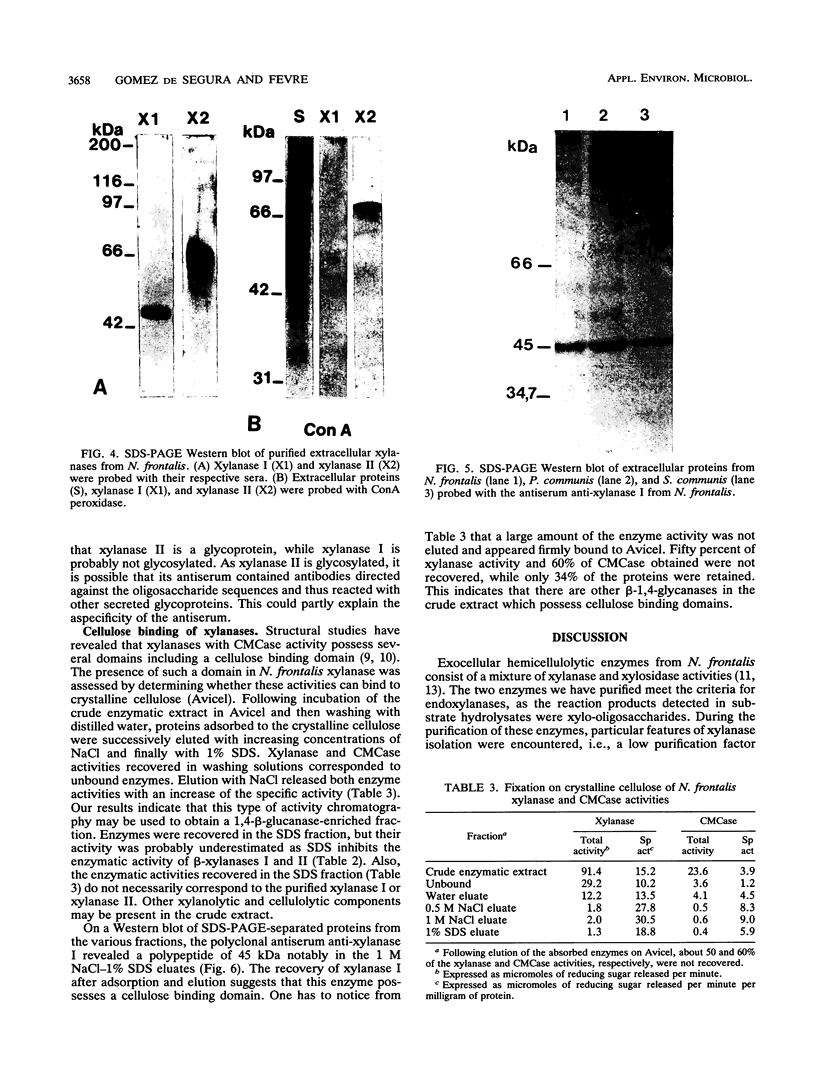
Two beta-endoxylanases produced by Neocallimastix frontalis have been purified by ammonium sulfate precipitation, gel filtration, and ion-exchange chromatography. Xylanase I is a nonglycosylated protein with an apparent molecular mass of 45 kDa. Xylanase II is a glycoprotein with an apparent molecular mass of 70 kDa. The pH optima of these enzymes were 5.5 and 6, respectively, and the temperature optimum was 55 degrees C for each enzyme. The endo mode of action of the enzymes was revealed by thin-layer chromatography of xylan hydrolysates. Antibodies raised against each purified protein exhibited no cross-reaction, confirming the biochemical specificities of the enzymes. Both enzymes exhibited carboxymethyl cellulase activity, and xylanase I was absorbed on crystalline cellulose, indicating that these enzymes might belong to the F family of beta-1,4-glycanases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Faye L., Chrispeels M. J. Characterization of N-linked oligosaccharides by affinoblotting with concanavalin A-peroxidase and treatment of the blots with glycosidases. Anal Biochem. 1985 Aug 15;149(1):218–224. doi: 10.1016/0003-2697(85)90498-1. [DOI] [PubMed] [Google Scholar]
- Gilkes N. R., Claeyssens M., Aebersold R., Henrissat B., Meinke A., Morrison H. D., Kilburn D. G., Warren R. A., Miller R. C., Jr Structural and functional relationships in two families of beta-1,4-glycanases. Eur J Biochem. 1991 Dec 5;202(2):367–377. doi: 10.1111/j.1432-1033.1991.tb16384.x. [DOI] [PubMed] [Google Scholar]
- Gilkes N. R., Henrissat B., Kilburn D. G., Miller R. C., Jr, Warren R. A. Domains in microbial beta-1, 4-glycanases: sequence conservation, function, and enzyme families. Microbiol Rev. 1991 Jun;55(2):303–315. doi: 10.1128/mr.55.2.303-315.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebraud M., Fevre M. Purification and Characterization of an Aspecific Glycoside Hydrolase from the Anaerobic Ruminal Fungus Neocallimastix frontalis. Appl Environ Microbiol. 1990 Oct;56(10):3164–3169. doi: 10.1128/aem.56.10.3164-3169.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebraud M., Fevre M. Purification and characterization of an extracellular beta-xylosidase from the rumen anaerobic fungus Neocallimastix frontalis. FEMS Microbiol Lett. 1990 Oct;60(1-2):11–16. doi: 10.1016/0378-1097(90)90336-o. [DOI] [PubMed] [Google Scholar]
- Henrissat B., Claeyssens M., Tomme P., Lemesle L., Mornon J. P. Cellulase families revealed by hydrophobic cluster analysis. Gene. 1989 Sep 1;81(1):83–95. doi: 10.1016/0378-1119(89)90339-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Mountfort D. O., Asher R. A. Production and regulation of cellulase by two strains of the rumen anaerobic fungus Neocallimastix frontalis. Appl Environ Microbiol. 1985 May;49(5):1314–1322. doi: 10.1128/aem.49.5.1314-1322.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer J. C., Nakas J. P. Purification and characterization of two xylanases from Trichoderma longibrachiatum. Eur J Biochem. 1991 Dec 5;202(2):521–529. doi: 10.1111/j.1432-1033.1991.tb16404.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. G., Orpin C. G. Polysaccharide-degrading enzymes formed by three species of anaerobic rumen fungi grown on a range of carbohydrate substrates. Can J Microbiol. 1987 May;33(5):418–426. doi: 10.1139/m87-071. [DOI] [PubMed] [Google Scholar]
- Xue G. P., Gobius K. S., Orpin C. G. A novel polysaccharide hydrolase cDNA (celD) from Neocallimastix patriciarum encoding three multi-functional catalytic domains with high endoglucanase, cellobiohydrolase and xylanase activities. J Gen Microbiol. 1992 Nov;138(11):2397–2403. doi: 10.1099/00221287-138-11-2397. [DOI] [PubMed] [Google Scholar]