Abstract
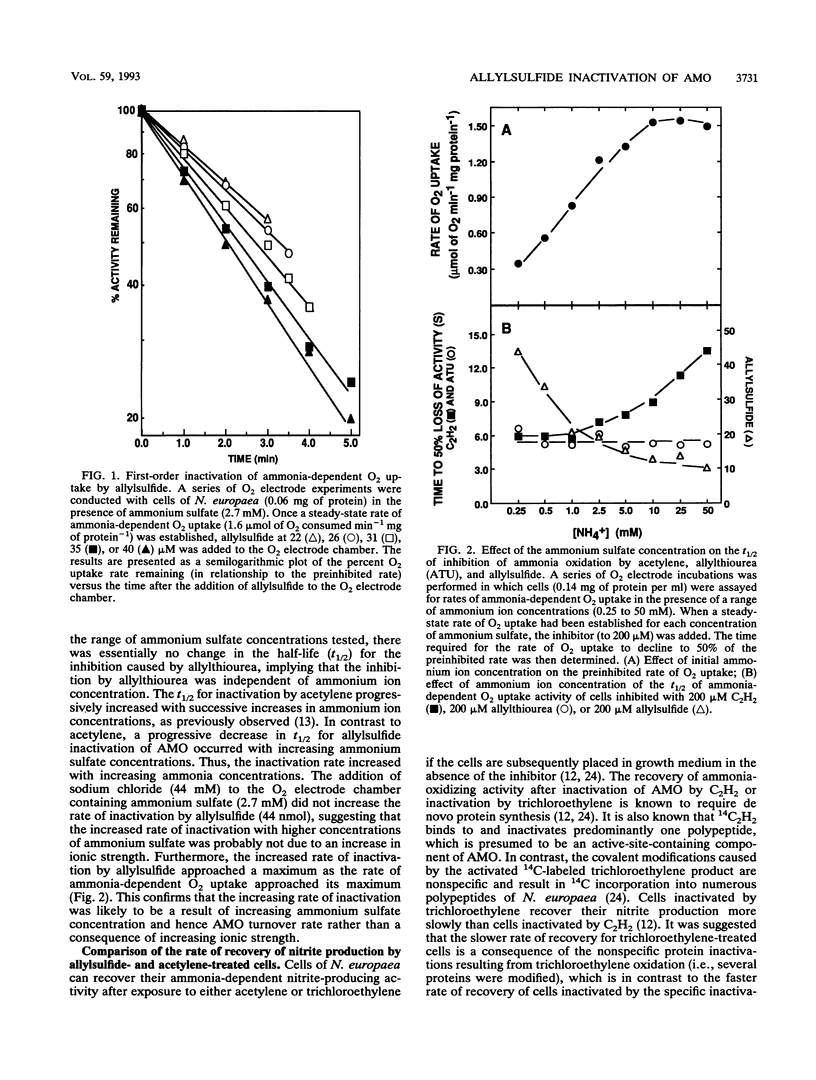
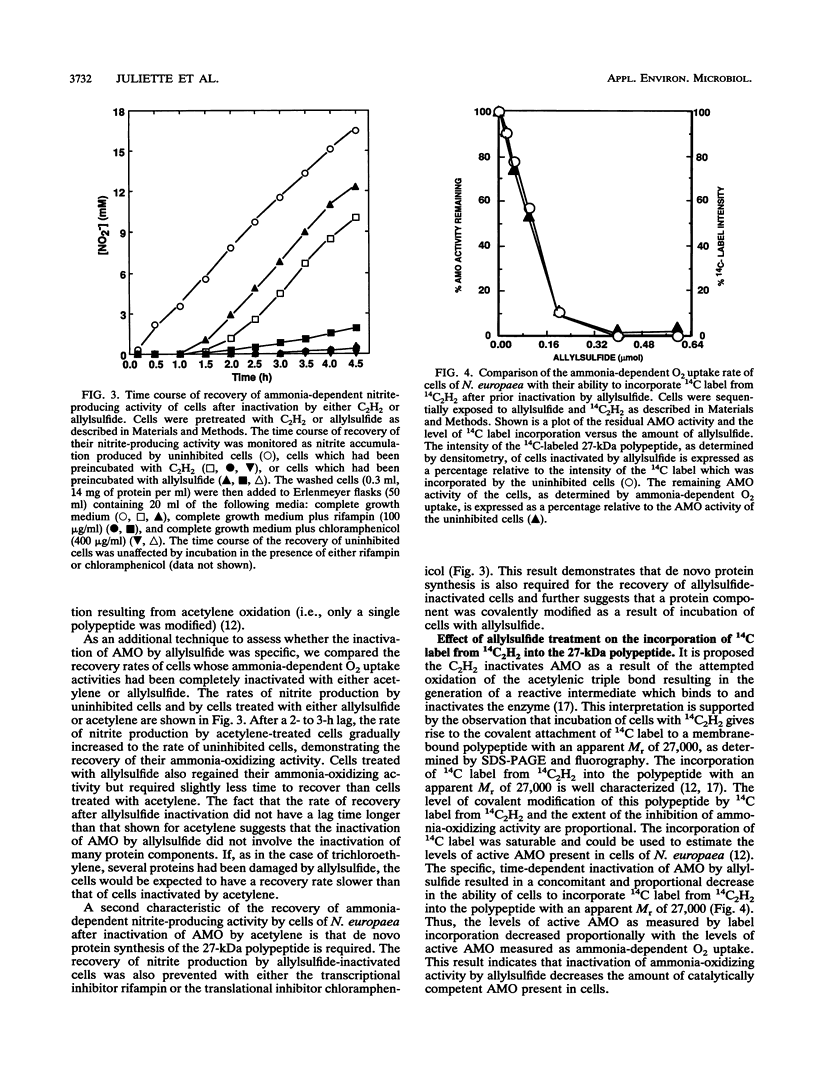
Allylsulfide caused an irreversible inactivation of ammonia monooxygenase (AMO) activity (ammonia-dependent O2 uptake) in Nitrosomonas europaea. The hydroxylamine oxidoreductase activity (hydrazine-dependent O2 uptake) of cells was unaffected by allylsulfide. Anaerobic conditions or the presence of allylthiourea, a reversible noncompetitive AMO inhibitor, protected AMO from inactivation by allylsulfide. Ammonia did not protect AMO from inactivation by allylsulfide but instead increased the rate of inactivation. The inactivation of AMO followed pseudo-first-order kinetics, but the observed rates did not saturate with increasing allylsulfide concentrations. The time course of recovery of AMO-dependent nitrite production after complete inactivation by allylsulfide required de novo protein synthesis. Incubation of cells with allylsulfide prevented the 14C label from 14C2H2 (a suicide mechanism-based inactivator of AMO) from being incorporated into the 27-kDa polypeptide of AMO. Some compounds structurally related to allylsulfide were unable to inactivate AMO. We conclude that allylsulfide is a specific, mechanism-based inactivator of AMO in N. europaea.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brady J. F., Ishizaki H., Fukuto J. M., Lin M. C., Fadel A., Gapac J. M., Yang C. S. Inhibition of cytochrome P-450 2E1 by diallyl sulfide and its metabolites. Chem Res Toxicol. 1991 Nov-Dec;4(6):642–647. doi: 10.1021/tx00024a008. [DOI] [PubMed] [Google Scholar]
- Brady J. F., Li D. C., Ishizaki H., Yang C. S. Effect of diallyl sulfide on rat liver microsomal nitrosamine metabolism and other monooxygenase activities. Cancer Res. 1988 Nov 1;48(21):5937–5940. [PubMed] [Google Scholar]
- HOFMAN T., LEES H. The biochemistry of the nitrifying organisms. IV. The respiration and intermediary metabolism of Nitrosomonas. Biochem J. 1953 Jul;54(4):579–583. doi: 10.1042/bj0540579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman M. R., Arp D. J. 14C2H2- and 14CO2-labeling studies of the de novo synthesis of polypeptides by Nitrosomonas europaea during recovery from acetylene and light inactivation of ammonia monooxygenase. J Biol Chem. 1992 Jan 25;267(3):1534–1545. [PubMed] [Google Scholar]
- Hyman M. R., Arp D. J. Acetylene inhibition of metalloenzymes. Anal Biochem. 1988 Sep;173(2):207–220. doi: 10.1016/0003-2697(88)90181-9. [DOI] [PubMed] [Google Scholar]
- Hyman M. R., Arp D. J. Quantification and removal of some contaminating gases from acetylene used to study gas-utilizing enzymes and microorganisms. Appl Environ Microbiol. 1987 Feb;53(2):298–303. doi: 10.1128/aem.53.2.298-303.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman M. R., Arp D. J. The small-scale production of [U-14C]acetylene from Ba14CO3: application to labeling of ammonia monooxygenase in autotrophic nitrifying bacteria. Anal Biochem. 1990 Nov 1;190(2):348–353. doi: 10.1016/0003-2697(90)90206-o. [DOI] [PubMed] [Google Scholar]
- Hyman M. R., Kim C. Y., Arp D. J. Inhibition of ammonia monooxygenase in Nitrosomonas europaea by carbon disulfide. J Bacteriol. 1990 Sep;172(9):4775–4782. doi: 10.1128/jb.172.9.4775-4782.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman M. R., Murton I. B., Arp D. J. Interaction of Ammonia Monooxygenase from Nitrosomonas europaea with Alkanes, Alkenes, and Alkynes. Appl Environ Microbiol. 1988 Dec;54(12):3187–3190. doi: 10.1128/aem.54.12.3187-3190.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman M. R., Wood P. M. Methane oxidation by Nitrosomonas europaea. Biochem J. 1983 Apr 15;212(1):31–37. doi: 10.1042/bj2120031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman M. R., Wood P. M. Suicidal inactivation and labelling of ammonia mono-oxygenase by acetylene. Biochem J. 1985 May 1;227(3):719–725. doi: 10.1042/bj2270719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliette L. Y., Hyman M. R., Arp D. J. Inhibition of Ammonia Oxidation in Nitrosomonas europaea by Sulfur Compounds: Thioethers Are Oxidized to Sulfoxides by Ammonia Monooxygenase. Appl Environ Microbiol. 1993 Nov;59(11):3718–3727. doi: 10.1128/aem.59.11.3718-3727.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Culbertson C. W. Evaluation of methyl fluoride and dimethyl ether as inhibitors of aerobic methane oxidation. Appl Environ Microbiol. 1992 Sep;58(9):2983–2992. doi: 10.1128/aem.58.9.2983-2992.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasche M. E., Hyman M. R., Arp D. J. Factors Limiting Aliphatic Chlorocarbon Degradation by Nitrosomonas europaea: Cometabolic Inactivation of Ammonia Monooxygenase and Substrate Specificity. Appl Environ Microbiol. 1991 Oct;57(10):2986–2994. doi: 10.1128/aem.57.10.2986-2994.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears J. H., Wood P. M. Spectroscopic evidence for a photosensitive oxygenated state of ammonia mono-oxygenase. Biochem J. 1985 Mar 1;226(2):499–507. doi: 10.1042/bj2260499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. M., 2nd, Hebbel R. P., Tukey D. P., Clawson C. C., White J. G., Vercellotti G. M. Pluronic F-68 reduces the endothelial adherence and improves the rheology of liganded sickle erythrocytes. Blood. 1987 Jun;69(6):1631–1636. [PubMed] [Google Scholar]
- Stotzky G., Schenck S. Volatile organic compounds and microorganisms. CRC Crit Rev Microbiol. 1976 May;4(4):333–382. doi: 10.3109/10408417609102303. [DOI] [PubMed] [Google Scholar]


