Abstract
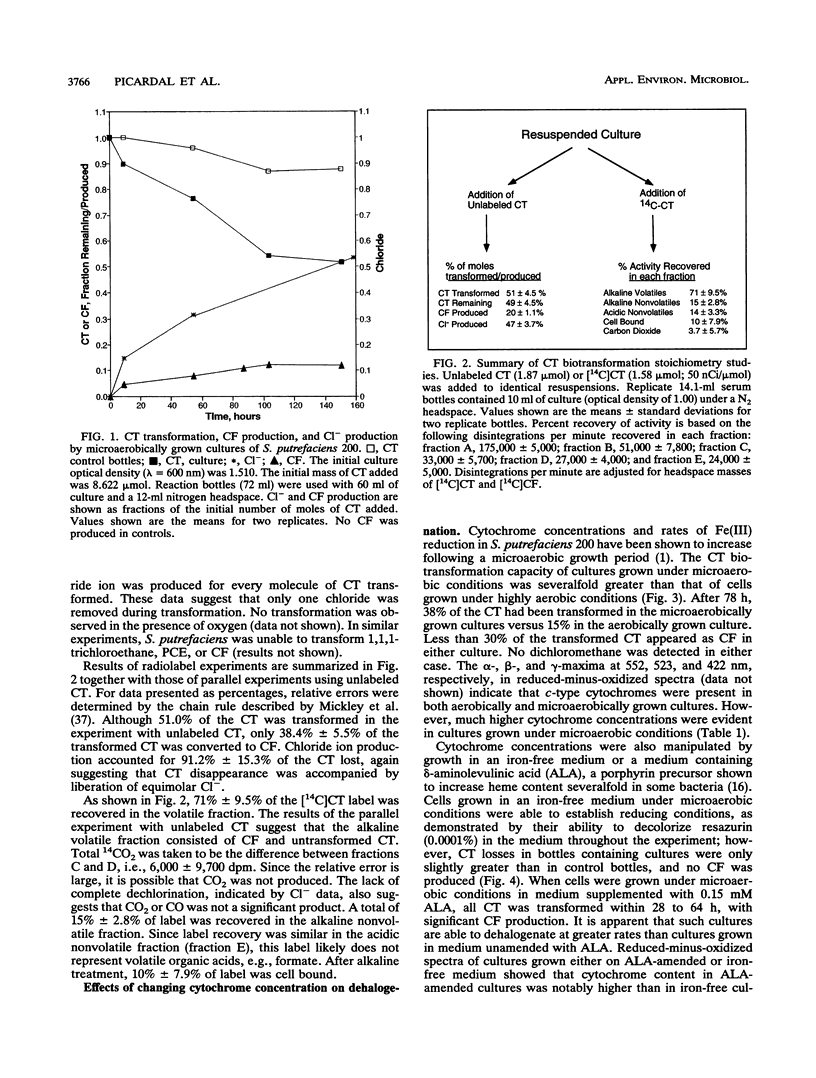
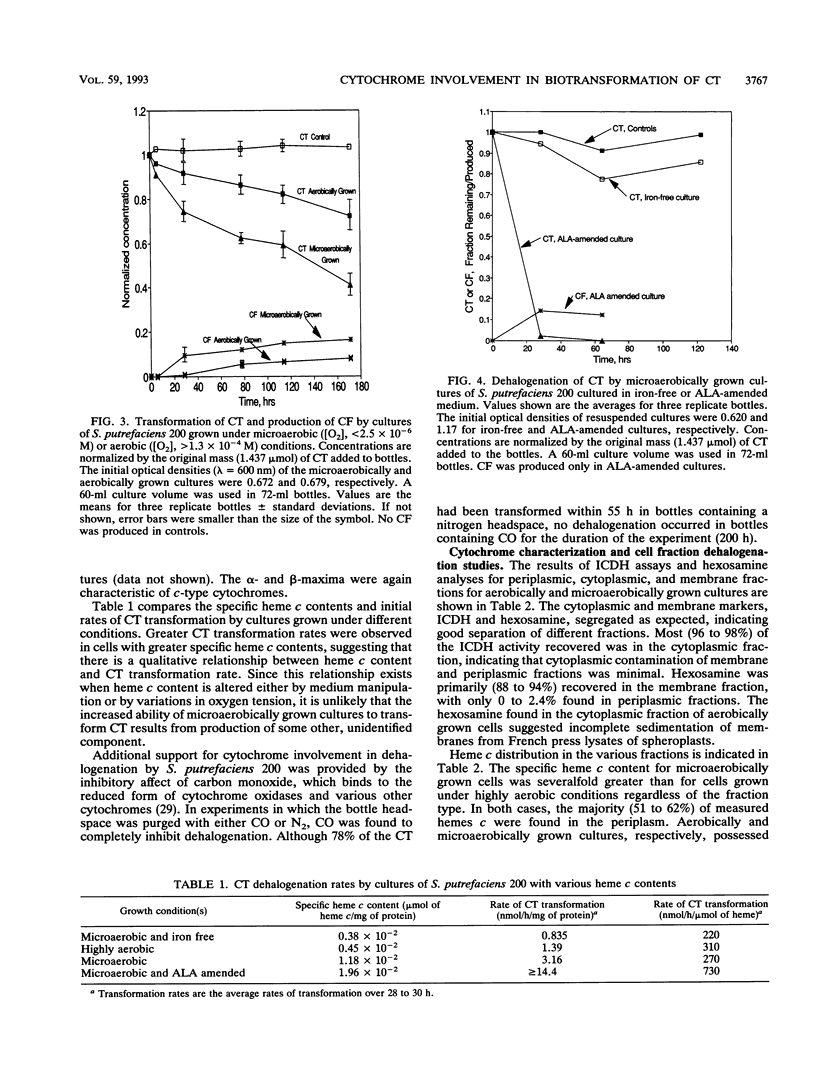
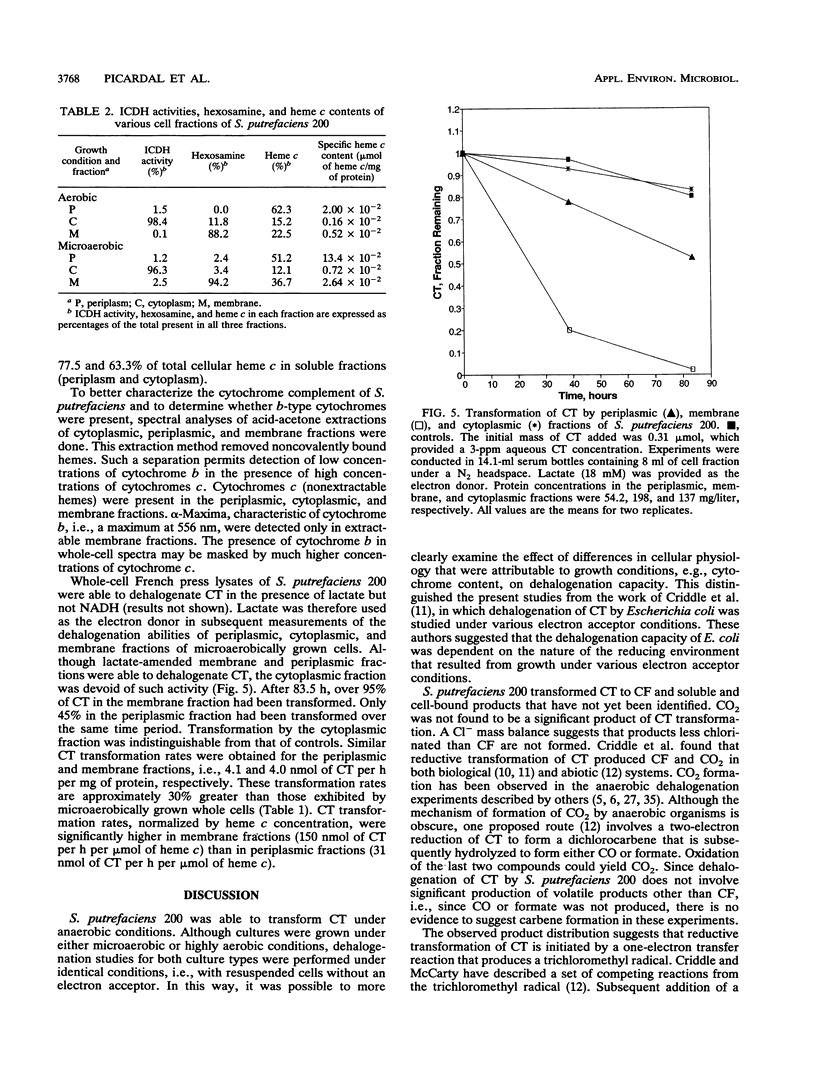
Shewanella putrefaciens 200 is an obligate respiratory bacterium that can utilize a variety of terminal electron acceptors, e.g., NO3-, NO2-, Fe(III), and trimethylamine N-oxide, in the absence of O2. The bacterium catalyzed the reductive transformation of tetrachloromethane (CT) under anaerobic conditions. The only identified product was trichloromethane (CF), but CF production was not stoichiometric. No dichloromethane, chloromethane, or methane was produced. A chloride mass balance indicated that fully dechlorinated products were not formed. Studies with [14C]CT suggested that a portion of the transformed CT reacted with biomass to form unidentified soluble and insoluble products. Intermediate production of a trichloromethyl radical can explain observed product distribution without significant CO2 formation. Evidence suggests that respiratory c-type cytochromes are responsible for the dehalogenation ability of S. putrefaciens 200. Previous growth under microaerobic conditions ([O2], < 2.5 microM) results in (i) a 2.6-fold increase in specific heme c content and (ii) a 2.3-fold increase in specific rates of anaerobic CT transformation. Manipulation of heme content by growth on iron-free medium or medium amended with delta-aminolevulinic acid showed that CT transformation rates increase with increases in specific heme c content. Transformation of CT is inhibited by CO. Dehalogenation studies with periplasmic, cytoplasmic, and membrane fractions indicated that only periplasmic and membrane fractions possessed dehalogenation ability. Cytochromes c were the predominant cytochromes present. Membranes were also found to contain smaller amounts of cytochrome b. Observed CT transformation patterns are consistent with a cometabolic description involving fortuitous CT reduction by reduced c-type cytochromes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold R. G., DiChristina T. J., Hoffmann M. R. Inhibitor studies of dissimilative Fe(III) reduction by Pseudomonas sp. strain 200 ("Pseudomonas ferrireductans") Appl Environ Microbiol. 1986 Aug;52(2):281–289. doi: 10.1128/aem.52.2.281-289.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf-Anid N., Nies L., Vogel T. M. Reductive dechlorination of a polychlorinated biphenyl congener and hexachlorobenzene by vitamin B12. Appl Environ Microbiol. 1992 Mar;58(3):1057–1060. doi: 10.1128/aem.58.3.1057-1060.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwer E. J., McCarty P. L. Transformations of 1- and 2-carbon halogenated aliphatic organic compounds under methanogenic conditions. Appl Environ Microbiol. 1983 Apr;45(4):1286–1294. doi: 10.1128/aem.45.4.1286-1294.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Castro C. E., Wade R. S., Belser N. O. Biodehalogenation: reactions of cytochrome P-450 with polyhalomethanes. Biochemistry. 1985 Jan 1;24(1):204–210. doi: 10.1021/bi00322a029. [DOI] [PubMed] [Google Scholar]
- Criddle C. S., DeWitt J. T., Grbić-Galić D., McCarty P. L. Transformation of carbon tetrachloride by Pseudomonas sp. strain KC under denitrification conditions. Appl Environ Microbiol. 1990 Nov;56(11):3240–3246. doi: 10.1128/aem.56.11.3240-3246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criddle C. S., DeWitt J. T., McCarty P. L. Reductive dehalogenation of carbon tetrachloride by Escherichia coli K-12. Appl Environ Microbiol. 1990 Nov;56(11):3247–3254. doi: 10.1128/aem.56.11.3247-3254.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWeerd K. A., Concannon F., Suflita J. M. Relationship between hydrogen consumption, dehalogenation, and the reduction of sulfur oxyanions by Desulfomonile tiedjei. Appl Environ Microbiol. 1991 Jul;57(7):1929–1934. doi: 10.1128/aem.57.7.1929-1934.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deweerd K. A., Suflita J. M. Anaerobic Aryl Reductive Dehalogenation of Halobenzoates by Cell Extracts of "Desulfomonile tiedjei". Appl Environ Microbiol. 1990 Oct;56(10):2999–3005. doi: 10.1128/aem.56.10.2999-3005.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolfing J. Reductive dechlorination of 3-chlorobenzoate is coupled to ATP production and growth in an anaerobic bacterium, strain DCB-1. Arch Microbiol. 1990;153(3):264–266. doi: 10.1007/BF00249079. [DOI] [PubMed] [Google Scholar]
- Doss M., Philipp-Dormston W. K. Regulatory link between lactate dehydrogenase and biosynthesis of porphyrin and heme in microorganisms. Enzyme. 1973;16(1):28–41. doi: 10.1159/000459359. [DOI] [PubMed] [Google Scholar]
- Egli C., Tschan T., Scholtz R., Cook A. M., Leisinger T. Transformation of tetrachloromethane to dichloromethane and carbon dioxide by Acetobacterium woodii. Appl Environ Microbiol. 1988 Nov;54(11):2819–2824. doi: 10.1128/aem.54.11.2819-2824.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gälli R., McCarty P. L. Biotransformation of 1,1,1-trichloroethane, trichloromethane, and tetrachloromethane by a Clostridium sp. Appl Environ Microbiol. 1989 Apr;55(4):837–844. doi: 10.1128/aem.55.4.837-844.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliger C., Kengen S. W., Schraa G., Stams A. J., Zehnder A. J. Methyl-coenzyme M reductase of Methanobacterium thermoautotrophicum delta H catalyzes the reductive dechlorination of 1,2-dichloroethane to ethylene and chloroethane. J Bacteriol. 1992 Jul;174(13):4435–4443. doi: 10.1128/jb.174.13.4435-4443.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliger C., Schraa G., Stupperich E., Stams A. J., Zehnder A. J. Evidence for the involvement of corrinoids and factor F430 in the reductive dechlorination of 1,2-dichloroethane by Methanosarcina barkeri. J Bacteriol. 1992 Jul;174(13):4427–4434. doi: 10.1128/jb.174.13.4427-4434.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krone U. E., Laufer K., Thauer R. K., Hogenkamp H. P. Coenzyme F430 as a possible catalyst for the reductive dehalogenation of chlorinated C1 hydrocarbons in methanogenic bacteria. Biochemistry. 1989 Dec 26;28(26):10061–10065. doi: 10.1021/bi00452a027. [DOI] [PubMed] [Google Scholar]
- Krone U. E., Thauer R. K., Hogenkamp H. P., Steinbach K. Reductive formation of carbon monoxide from CCl4 and FREONs 11, 12, and 13 catalyzed by corrinoids. Biochemistry. 1991 Mar 12;30(10):2713–2719. doi: 10.1021/bi00224a020. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Studies of the electron transport chain of extremely halophilic bacteria. I. Spectrophotometric identification of the cytochromes of Halobacterium cutirubrum. Arch Biochem Biophys. 1968 Dec;128(3):716–724. doi: 10.1016/0003-9861(68)90080-5. [DOI] [PubMed] [Google Scholar]
- Linkfield T. G., Tiedje J. M. Characterization of the requirements and substrates for reductive dehalogenation by strain DCB-1. J Ind Microbiol. 1990 Jan;5(1):9–15. doi: 10.1007/BF01569601. [DOI] [PubMed] [Google Scholar]
- Meyer T. E., Przysiecki C. T., Watkins J. A., Bhattacharyya A., Simondsen R. P., Cusanovich M. A., Tollin G. Correlation between rate constant for reduction and redox potential as a basis for systematic investigation of reaction mechanisms of electron transfer proteins. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6740–6744. doi: 10.1073/pnas.80.22.6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. J., Gibson D. M., Ward F. B. Influence of respiratory substrate on the cytochrome content of Shewanella putrefaciens. FEMS Microbiol Lett. 1990 Jun 1;57(3):259–262. doi: 10.1016/0378-1097(90)90077-4. [DOI] [PubMed] [Google Scholar]
- Myers C. R., Myers J. M. Localization of cytochromes to the outer membrane of anaerobically grown Shewanella putrefaciens MR-1. J Bacteriol. 1992 Jun;174(11):3429–3438. doi: 10.1128/jb.174.11.3429-3438.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obuekwe C. O., Westlake D. W., Cook F. D. Effect of nitrate on reduction of ferric iron by a bacterium isolated from crude oil. Can J Microbiol. 1981 Jul;27(7):692–697. doi: 10.1139/m81-107. [DOI] [PubMed] [Google Scholar]
- Obuekwe C. O., Westlake D. W. Effects of medium composition on cell pigmentation, cytochrome content, and ferric iron reduction in a Pseudomonas sp. isolated from crude oil. Can J Microbiol. 1982 Aug;28(8):989–992. doi: 10.1139/m82-148. [DOI] [PubMed] [Google Scholar]


