Abstract
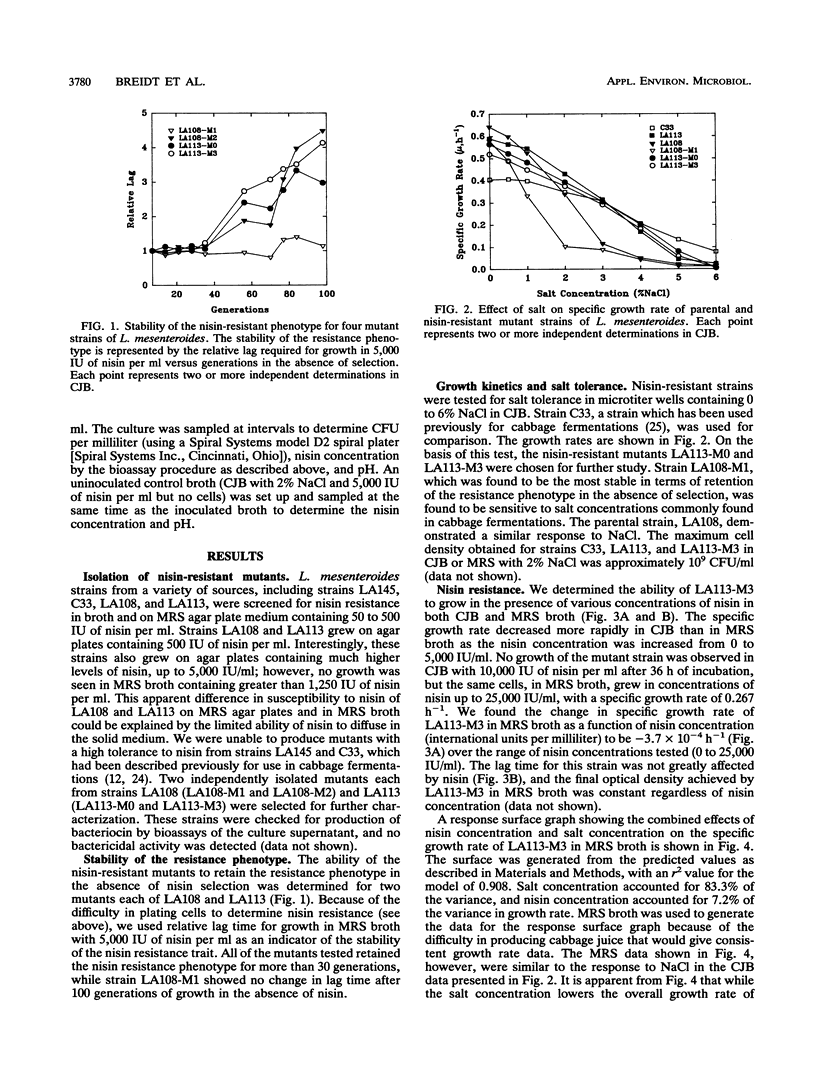
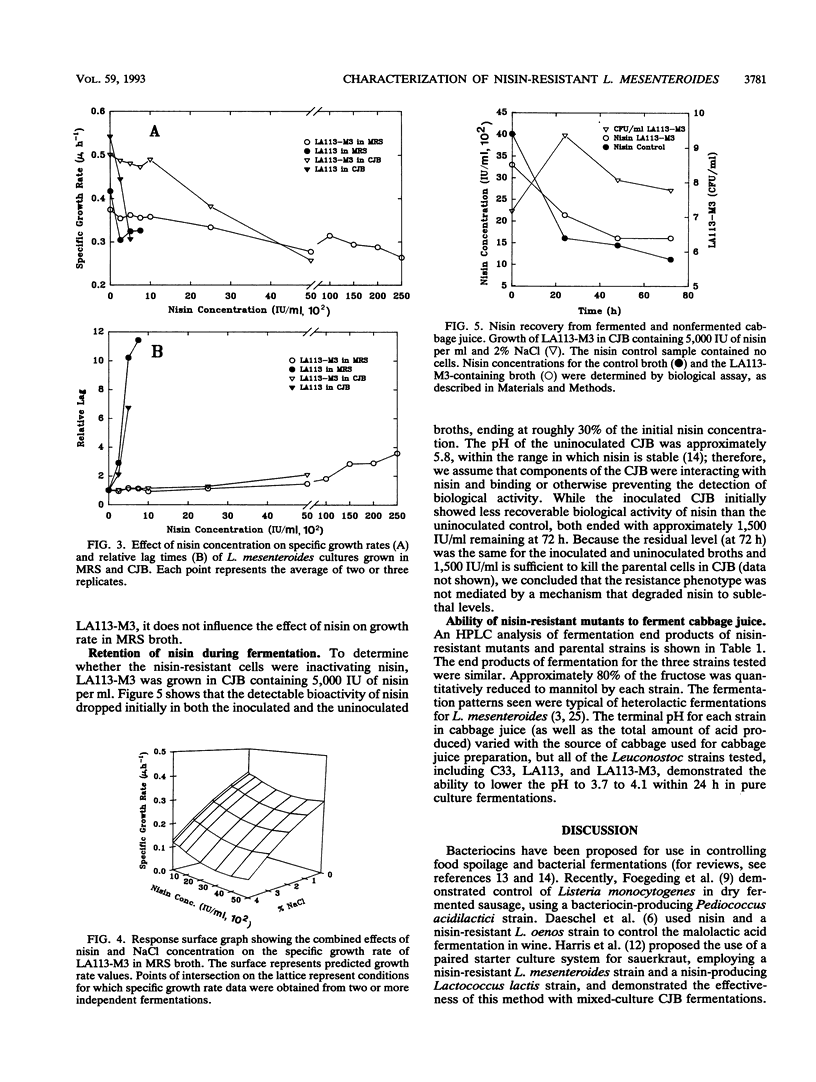
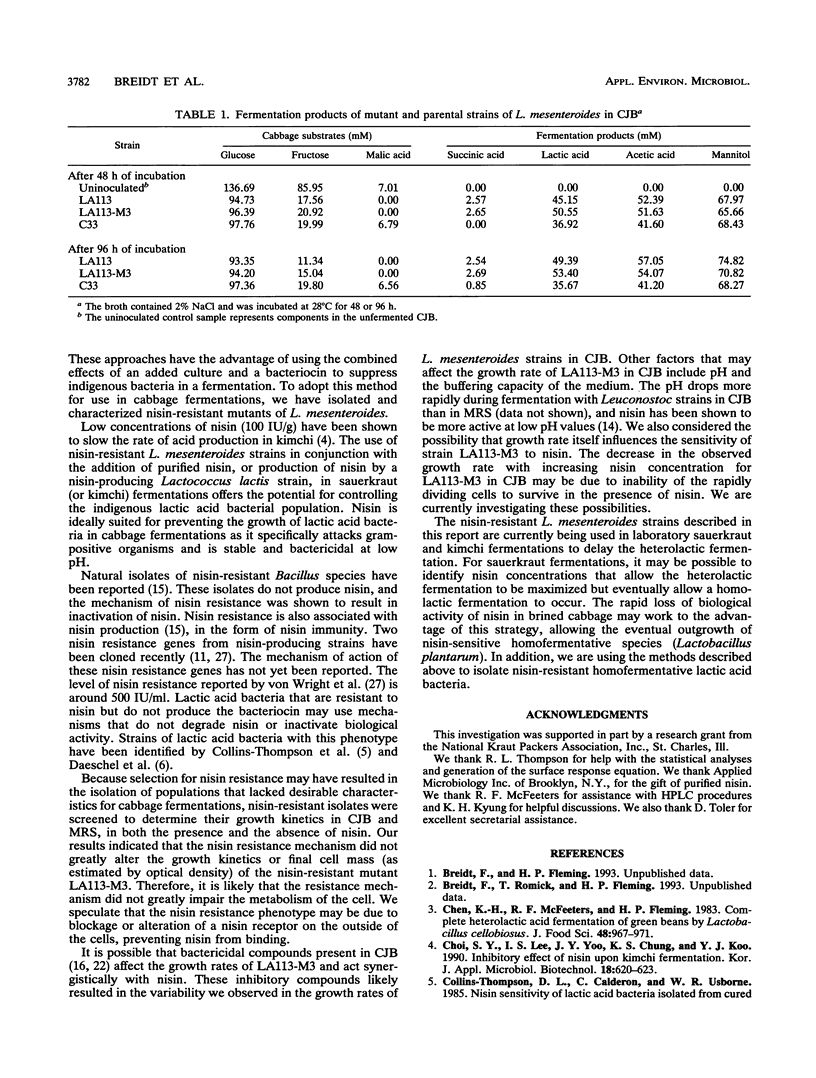
Leuconostoc mesenteroides strains that are resistant to high levels of nisin (up to 25,000 IU/ml in broth) were isolated. These nisin-resistant mutants were evaluated to determine their potential use as starter culture strains for cabbage fermentations. We found that some L. mesenteroides strains could be adapted to high levels of nisin resistance, while others could not. The nisin resistance trait was found to be stable for at least 35 generations, in the absence of nisin selection, for all mutants tested. The effects of nisin and salt, separately and in combination, on growth kinetics of the nisin-resistant strains were determined. Salt was the most influential factor on the specific growth rates of the mutants, and no synergistic effect between nisin and salt on specific growth rates was observed. The nisin-resistant strains were unimpaired in their ability to rapidly produce normal heterolactic fermentation end products. The use of these L. mesenteroides mutants as starter cultures in combination with nisin may extend the heterolactic phase of cabbage fermentations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daeschel M. A., Jung D. S., Watson B. T. Controlling Wine Malolactic Fermentation with Nisin and Nisin-Resistant Strains of Leuconostoc oenos. Appl Environ Microbiol. 1991 Feb;57(2):601–603. doi: 10.1128/aem.57.2.601-603.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foegeding P. M., Thomas A. B., Pilkington D. H., Klaenhammer T. R. Enhanced control of Listeria monocytogenes by in situ-produced pediocin during dry fermented sausage production. Appl Environ Microbiol. 1992 Mar;58(3):884–890. doi: 10.1128/aem.58.3.884-890.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froseth B. R., Herman R. E., McKay L. L. Cloning of nisin resistance determinant and replication origin on 7.6-kilobase EcoRI fragment of pNP40 from Streptococcus lactis subsp. diacetylactis DRC3. Appl Environ Microbiol. 1988 Aug;54(8):2136–2139. doi: 10.1128/aem.54.8.2136-2139.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris L. J., Fleming H. P., Klaenhammer T. R. Novel paired starter culture system for sauerkraut, consisting of a nisin-resistant Leuconostoc mesenteroides strain and a nisin-producing Lactococcus lactis strain. Appl Environ Microbiol. 1992 May;58(5):1484–1489. doi: 10.1128/aem.58.5.1484-1489.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis B. Resistance to nisin and production of nisin-inactivating enzymes by several Bacillus species. J Gen Microbiol. 1967 Apr;47(1):33–48. doi: 10.1099/00221287-47-1-33. [DOI] [PubMed] [Google Scholar]
- von Wright A., Wessels S., Tynkkynen S., Saarela M. Isolation of a replication region of a large lactococcal plasmid and use in cloning of a nisin resistance determinant. Appl Environ Microbiol. 1990 Jul;56(7):2029–2035. doi: 10.1128/aem.56.7.2029-2035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


