Abstract
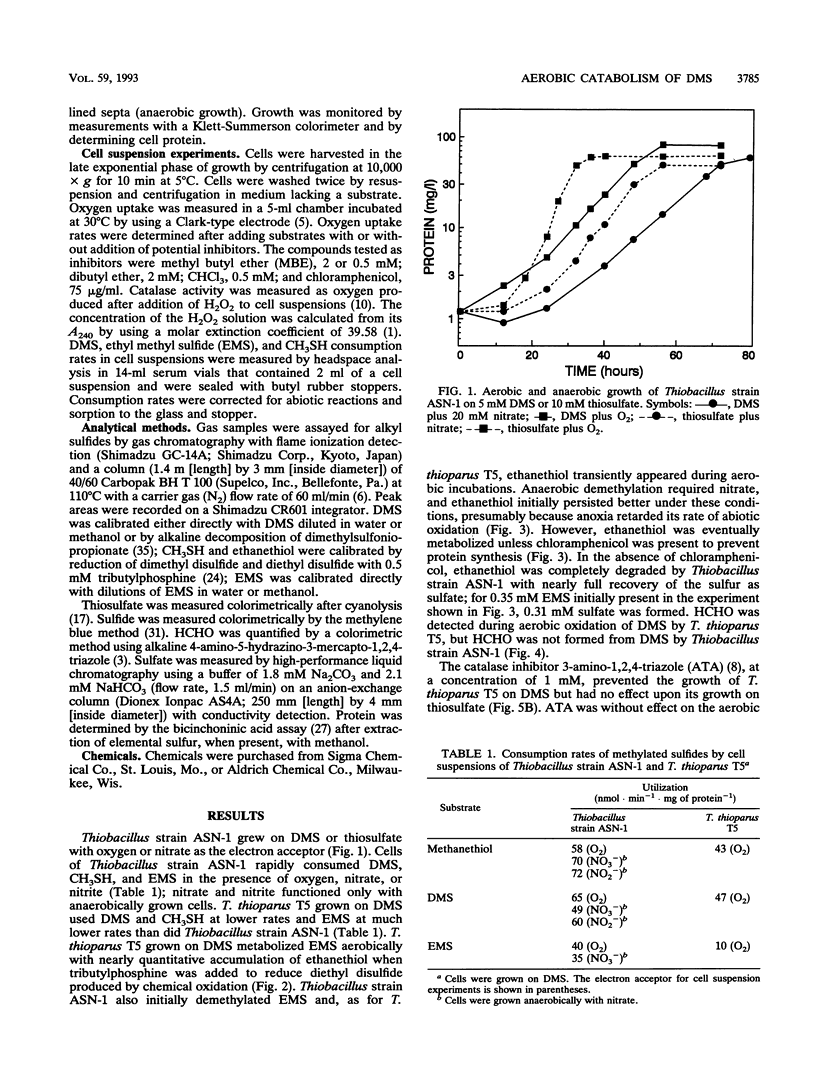
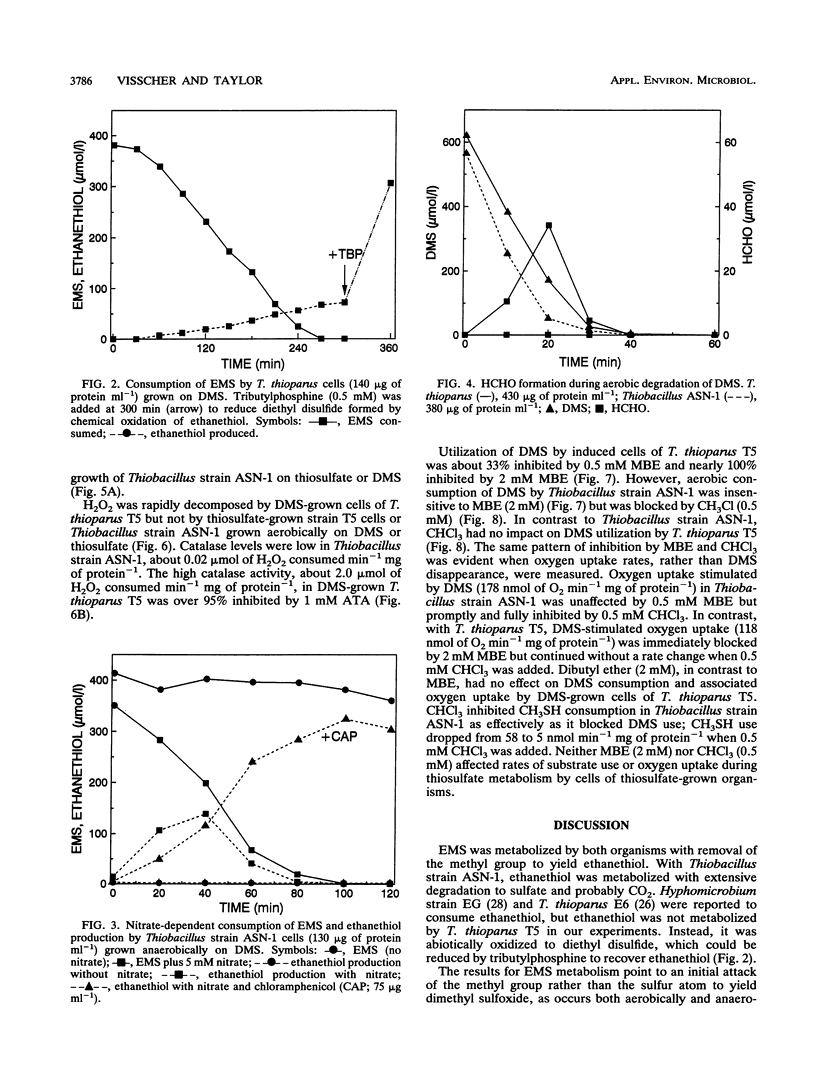
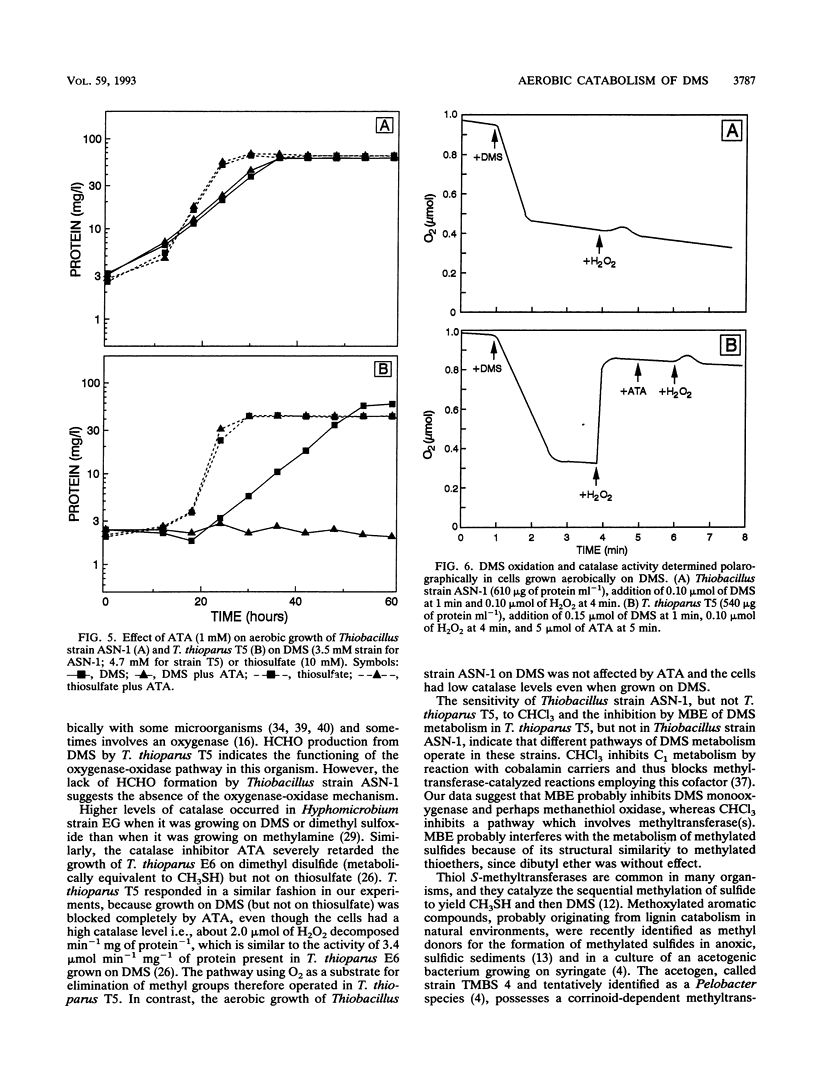
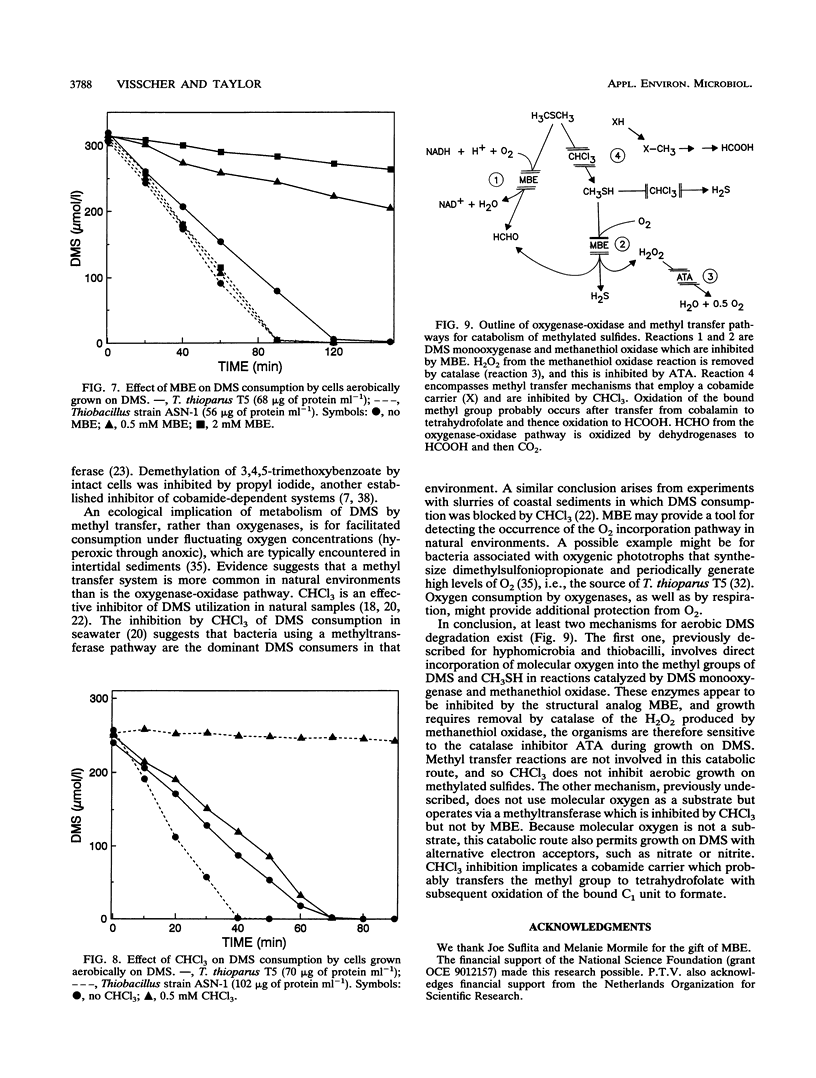
Aerobic degradation of dimethyl sulfide (DMS), previously described for thiobacilli and hyphomicrobia, involves catabolism to sulfide via methanethiol (CH3SH). Methyl groups are sequentially eliminated as HCHO by incorporation of O2 catalyzed by DMS monooxygenase and methanethiol oxidase. H2O2 formed during CH3SH oxidation is destroyed by catalase. We recently isolated Thiobacillus strain ASN-1, which grows either aerobically or anaerobically with denitrification on DMS. Comparative experiments with Thiobacillus thioparus T5, which grows only aerobically on DMS, indicate a novel mechanism for aerobic DMS catabolism by Thiobacillus strain ASN-1. Evidence that both organisms initially attacked the methyl group, rather than the sulfur atom, in DMS was their conversion of ethyl methyl sulfide to ethanethiol. HCHO transiently accumulated during the aerobic use of DMS by T. thioparus but not with Thiobacillus strain ASN-1. Catalase levels in cells grown aerobically on DMS were about 100-fold lower in Thiobacillus strain ASN-1 than in T. thioparus T5, suggesting the absence of H2O2 formation during DMS catabolism. Also, aerobic growth of T. thioparus T5 on DMS was blocked by the catalase inhibitor 3-amino-1,2,4-triazole whereas that of Thiobacillus strain ASN-1 was not. Methyl butyl ether, but not CHCl3, blocked DMS catabolism by T. thioparus T5, presumably by inhibiting DMS monooxygenase and perhaps methanethiol oxidase. In contrast, DMS metabolism by Thiobacillus strain ASN-1 was unaffected by methyl butyl ether but inhibited by CHCl3. DMS catabolism by Thiobacillus strain ASN-1 probably involves methyl transfer to a cobalamin carrier and subsequent oxidation as folate-bound intermediates.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Avigad G. A simple spectrophotometric determination of formaldehyde and other aldehydes: application to periodate-oxidized glycol systems. Anal Biochem. 1983 Oct 15;134(2):499–504. doi: 10.1016/0003-2697(83)90330-5. [DOI] [PubMed] [Google Scholar]
- BROT N., WEISSBACH H. ENZYMATIC SYNTHESIS OF METHIONINE. CHEMICAL ALKYLATION OF THE ENZYME-BOUND COBAMIDE. J Biol Chem. 1965 Jul;240:3064–3070. [PubMed] [Google Scholar]
- Cohen G., Somerson N. L. Catalase-aminotriazole method for measuring secretion of hydrogen peroxide by microorganisms. J Bacteriol. 1969 May;98(2):543–546. doi: 10.1128/jb.98.2.543-546.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Río L. A., Ortega M. G., López A. L., Gorgé J. L. A more sensitive modification of the catalase assay with the Clark oxygen electrode. Application to the kinetic study of the pea leaf enzyme. Anal Biochem. 1977 Jun;80(2):409–415. doi: 10.1016/0003-2697(77)90662-5. [DOI] [PubMed] [Google Scholar]
- Drotar A., Burton G. A., Jr, Tavernier J. E., Fall R. Widespread occurrence of bacterial thiol methyltransferases and the biogenic emission of methylated sulfur gases. Appl Environ Microbiol. 1987 Jul;53(7):1626–1631. doi: 10.1128/aem.53.7.1626-1631.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiene R. P., Oremland R. S., Catena A., Miller L. G., Capone D. G. Metabolism of reduced methylated sulfur compounds in anaerobic sediments and by a pure culture of an estuarine methanogen. Appl Environ Microbiol. 1986 Nov;52(5):1037–1045. doi: 10.1128/aem.52.5.1037-1045.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiene R. P., Visscher P. T. Production and fate of methylated sulfur compounds from methionine and dimethylsulfoniopropionate in anoxic salt marsh sediments. Appl Environ Microbiol. 1987 Oct;53(10):2426–2434. doi: 10.1128/aem.53.10.2426-2434.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni S. S., Boone D. R. Isolation and characterization of a dimethyl sulfide-degrading methanogen, Methanolobus siciliae HI350, from an oil well, characterization of M. siciliae T4/MT, and emendation of M. siciliae. Int J Syst Bacteriol. 1991 Jul;41(3):410–416. doi: 10.1099/00207713-41-3-410. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- TRUEPER H. G., SCHLEGEL H. G. SULPHUR METABOLISM IN THIORHODACEAE. I. QUANTITATIVE MEASUREMENTS ON GROWING CELLS OF CHROMATIUM OKENII. Antonie Van Leeuwenhoek. 1964;30:225–238. doi: 10.1007/BF02046728. [DOI] [PubMed] [Google Scholar]
- Visscher P. T., Quist P., van Gemerden H. Methylated sulfur compounds in microbial mats: in situ concentrations and metabolism by a colorless sulfur bacterium. Appl Environ Microbiol. 1991 Jun;57(6):1758–1763. doi: 10.1128/aem.57.6.1758-1763.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher P. T., van Gemerden H. Production and consumption of dimethylsulfoniopropionate in marine microbial mats. Appl Environ Microbiol. 1991 Nov;57(11):3237–3242. doi: 10.1128/aem.57.11.3237-3242.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdel F., Pfennig N. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. I. Isolation of new sulfate-reducing bacteria enriched with acetate from saline environments. Description of Desulfobacter postgatei gen. nov., sp. nov. Arch Microbiol. 1981 Jul;129(5):395–400. doi: 10.1007/BF00406470. [DOI] [PubMed] [Google Scholar]
- Wood J. M., Kennedy F. S., Wolfe R. S. The reaction of multihalogenated hydrocarbons with free and bound reduced vitamin B 12. Biochemistry. 1968 May;7(5):1707–1713. doi: 10.1021/bi00845a013. [DOI] [PubMed] [Google Scholar]
- Wood J. M., Wolfe R. S. Propylation and purification of a B12 enzyme involved in methane formation. Biochemistry. 1966 Nov;5(11):3598–3603. doi: 10.1021/bi00875a031. [DOI] [PubMed] [Google Scholar]
- Zeyer J., Eicher P., Wakeham S. G., Schwarzenbach R. P. Oxidation of dimethyl sulfide to dimethyl sulfoxide by phototrophic purple bacteria. Appl Environ Microbiol. 1987 Sep;53(9):2026–2032. doi: 10.1128/aem.53.9.2026-2032.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


