Abstract
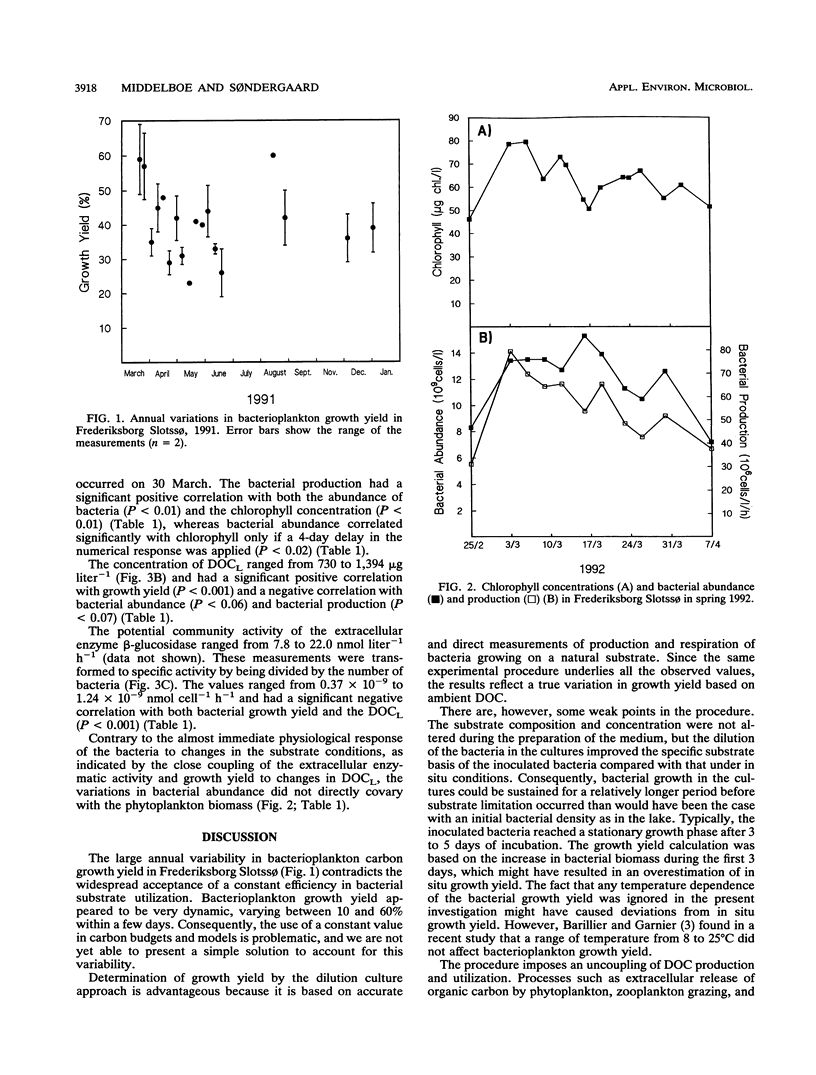
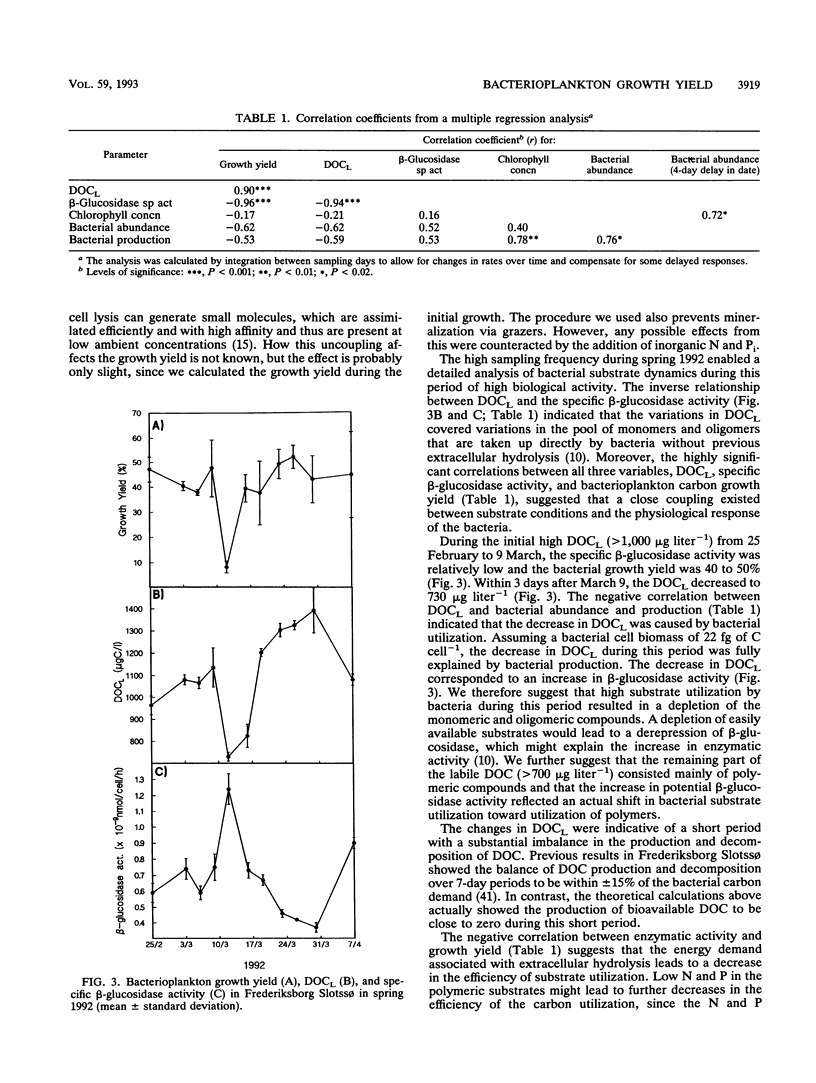
The seasonal variation in the carbon growth yield of pelagic bacteria in the eutrophic lake Frederiksborg Slotssø was studied. The growth yield was determined in dilution culture experiments, in which a substrate of dissolved organic carbon (DOC) from the lake was incubated with a natural bacterioplankton assemblage. Bacterial growth efficiency varied annually from 8 to 60% with an average (and standard deviation) of 41 ± 11% (n = 29). Simultaneous measurements of growth yield, substrate lability (DOCL), chlorophyll and bacterial production, abundance, and extracellular enzymatic activity revealed new aspects of the regulation of bacterial DOC utilization. Growth yield correlated positively to DOCL and negatively to β-d-glucosidase activity. These results indicated a close coupling between the substrate conditions and the physiological response of the bacteria. The large variations in yield within a few days and the close coupling to substrate availability showed that one single global carbon yield factor cannot be expected to apply in pelagic systems.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barillier A., Garnier J. Influence of temperature and substrate concentration on bacterial growth yield in Seine River water batch cultures. Appl Environ Microbiol. 1993 May;59(5):1678–1682. doi: 10.1128/aem.59.5.1678-1682.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducklow H. W., Purdie D. A., Williams P. J., Davies J. M. Bacterioplankton: a sink for carbon in a coastal marine plankton community. Science. 1986 May 16;232(4752):865–867. doi: 10.1126/science.232.4752.865. [DOI] [PubMed] [Google Scholar]
- Fuhrman J. A., Azam F. Bacterioplankton secondary production estimates for coastal waters of british columbia, antarctica, and california. Appl Environ Microbiol. 1980 Jun;39(6):1085–1095. doi: 10.1128/aem.39.6.1085-1095.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempfling W. P., Mainzer S. E. Effects of varying the carbon source limiting growth on yield and maintenance characteristics of Escherichia coli in continuous culture. J Bacteriol. 1975 Sep;123(3):1076–1087. doi: 10.1128/jb.123.3.1076-1087.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe Hans-Georg, Kim Sang-Jin, Gocke Klaus. Microbial Decomposition in Aquatic Environments: Combined Process of Extracellular Enzyme Activity and Substrate Uptake. Appl Environ Microbiol. 1988 Mar;54(3):784–790. doi: 10.1128/aem.54.3.784-790.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirt S. J. Maintenance energy: a general model for energy-limited and energy-sufficient growth. Arch Microbiol. 1982 Dec 3;133(4):300–302. doi: 10.1007/BF00521294. [DOI] [PubMed] [Google Scholar]
- Smits J. D., Riemann B. Calculation of cell production from [h]thymidine incorporation with freshwater bacteria. Appl Environ Microbiol. 1988 Sep;54(9):2213–2219. doi: 10.1128/aem.54.9.2213-2219.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempest D. W., Neijssel O. M. The status of YATP and maintenance energy as biologically interpretable phenomena. Annu Rev Microbiol. 1984;38:459–486. doi: 10.1146/annurev.mi.38.100184.002331. [DOI] [PubMed] [Google Scholar]
- Tranvik L. J., Höfle M. G. Bacterial growth in mixed cultures on dissolved organic carbon from humic and clear waters. Appl Environ Microbiol. 1987 Mar;53(3):482–488. doi: 10.1128/aem.53.3.482-488.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


