Abstract
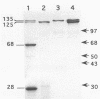
The cryIVA and cryIVB genes, encoding the 125- and 135-kDa proteins, respectively, of Bacillus thuringiensis subsp. israelensis, were cloned either alone or together into a shuttle vector and expressed in a nontoxic strain of B. thuringiensis subsp. israelensis. The CryIVB protein was produced at a high level during sporulation and accumulated as inclusions; in contrast, the CryIVA polypeptide did not form such structures unless it was cloned on a higher-copy-number plasmid. Transcriptional fusions between the cryIVA or cryIVB gene promoter and the lacZ gene were constructed. The poor synthesis of CryIVA was not due to a poor efficiency of transcription from the cryIVA gene promoter. Mosquitocidal assays performed with purified inclusions showed that CryIVA was toxic for larvae of the species Aedes aegypti, Anopheles stephensi, and Culex pipiens, whereas CryIVB displayed activity only toward Aedes aegypti and Anopheles stephensi. The activity of inclusions containing both polypeptides was higher than that of single-peptide inclusions but was not as high as that of the native crystals, which contain at least four polypeptides.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. F., Visick J. E., Whiteley H. R. A 20-kilodalton protein is required for efficient production of the Bacillus thuringiensis subsp. israelensis 27-kilodalton crystal protein in Escherichia coli. J Bacteriol. 1989 Jan;171(1):521–530. doi: 10.1128/jb.171.1.521-530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angsuthanasombat C., Crickmore N., Ellar D. J. Comparison of Bacillus thuringiensis subsp. israelensis CryIVA and CryIVB cloned toxins reveals synergism in vivo. FEMS Microbiol Lett. 1992 Jul 1;73(1-2):63–68. doi: 10.1016/0378-1097(92)90584-b. [DOI] [PubMed] [Google Scholar]
- Arantes O., Lereclus D. Construction of cloning vectors for Bacillus thuringiensis. Gene. 1991 Dec 1;108(1):115–119. doi: 10.1016/0378-1119(91)90495-w. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgouin C., Delécluse A., Ribier J., Klier A., Rapoport G. A Bacillus thuringiensis subsp. israelensis gene encoding a 125-kilodalton larvicidal polypeptide is associated with inverted repeat sequences. J Bacteriol. 1988 Aug;170(8):3575–3583. doi: 10.1128/jb.170.8.3575-3583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgouin C., Klier A., Rapoport G. Characterization of the genes encoding the haemolytic toxin and the mosquitocidal delta-endotoxin of Bacillus thuringiensis israelensis. Mol Gen Genet. 1986 Dec;205(3):390–397. doi: 10.1007/BF00338072. [DOI] [PubMed] [Google Scholar]
- Brown K. L., Whiteley H. R. Isolation of a Bacillus thuringiensis RNA polymerase capable of transcribing crystal protein genes. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4166–4170. doi: 10.1073/pnas.85.12.4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. L., Whiteley H. R. Isolation of the second Bacillus thuringiensis RNA polymerase that transcribes from a crystal protein gene promoter. J Bacteriol. 1990 Dec;172(12):6682–6688. doi: 10.1128/jb.172.12.6682-6688.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Dai S. M., Frutos R., Federici B. A., Gill S. S. Properties of a 72-kilodalton mosquitocidal protein from Bacillus thuringiensis subsp. morrisoni PG-14 expressed in B. thuringiensis subsp. kurstaki by using the shuttle vector pHT3101. Appl Environ Microbiol. 1992 Feb;58(2):507–512. doi: 10.1128/aem.58.2.507-512.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crickmore N., Ellar D. J. Involvement of a possible chaperonin in the efficient expression of a cloned CryIIA delta-endotoxin gene in Bacillus thuringiensis. Mol Microbiol. 1992 Jun;6(11):1533–1537. doi: 10.1111/j.1365-2958.1992.tb00874.x. [DOI] [PubMed] [Google Scholar]
- Debarbouille M., Arnaud M., Fouet A., Klier A., Rapoport G. The sacT gene regulating the sacPA operon in Bacillus subtilis shares strong homology with transcriptional antiterminators. J Bacteriol. 1990 Jul;172(7):3966–3973. doi: 10.1128/jb.172.7.3966-3973.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delécluse A., Bourgouin C., Klier A., Rapoport G. Specificity of action on mosquito larvae of Bacillus thuringiensis israelensis toxins encoded by two different genes. Mol Gen Genet. 1988 Sep;214(1):42–47. doi: 10.1007/BF00340177. [DOI] [PubMed] [Google Scholar]
- Delécluse A., Charles J. F., Klier A., Rapoport G. Deletion by in vivo recombination shows that the 28-kilodalton cytolytic polypeptide from Bacillus thuringiensis subsp. israelensis is not essential for mosquitocidal activity. J Bacteriol. 1991 Jun;173(11):3374–3381. doi: 10.1128/jb.173.11.3374-3381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González J. M., Jr, Carlton B. C. A large transmissible plasmid is required for crystal toxin production in Bacillus thuringiensis variety israelensis. Plasmid. 1984 Jan;11(1):28–38. doi: 10.1016/0147-619x(84)90004-0. [DOI] [PubMed] [Google Scholar]
- Höfte H., Whiteley H. R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev. 1989 Jun;53(2):242–255. doi: 10.1128/mr.53.2.242-255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecadet M. M., Blondel M. O., Ribier J. Generalized transduction in Bacillus thuringiensis var. berliner 1715 using bacteriophage CP-54Ber. J Gen Microbiol. 1980 Nov;121(1):203–212. doi: 10.1099/00221287-121-1-203. [DOI] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lereclus D., Arantès O., Chaufaux J., Lecadet M. Transformation and expression of a cloned delta-endotoxin gene in Bacillus thuringiensis. FEMS Microbiol Lett. 1989 Jul 15;51(1):211–217. doi: 10.1016/0378-1097(89)90511-9. [DOI] [PubMed] [Google Scholar]
- McLean K. M., Whiteley H. R. Expression in Escherichia coli of a cloned crystal protein gene of Bacillus thuringiensis subsp. israelensis. J Bacteriol. 1987 Mar;169(3):1017–1023. doi: 10.1128/jb.169.3.1017-1023.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabashnik B. E. Evaluation of synergism among Bacillus thuringiensis toxins. Appl Environ Microbiol. 1992 Oct;58(10):3343–3346. doi: 10.1128/aem.58.10.3343-3346.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas W. E., Ellar D. J. Bacillus thuringiensis var israelensis crystal delta-endotoxin: effects on insect and mammalian cells in vitro and in vivo. J Cell Sci. 1983 Mar;60:181–197. doi: 10.1242/jcs.60.1.181. [DOI] [PubMed] [Google Scholar]
- Visick J. E., Whiteley H. R. Effect of a 20-kilodalton protein from Bacillus thuringiensis subsp. israelensis on production of the CytA protein by Escherichia coli. J Bacteriol. 1991 Mar;173(5):1748–1756. doi: 10.1128/jb.173.5.1748-1756.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H. C., Schnepf H. E., Whiteley H. R. Transcriptional and translational start sites for the Bacillus thuringiensis crystal protein gene. J Biol Chem. 1983 Feb 10;258(3):1960–1967. [PubMed] [Google Scholar]