Abstract
Genomic probes were used to investigate hepatitis A virus (HAV) and enterovirus RNAs in two types of shellfish from natural beds (Atlantic coast, France). After elution concentration, nucleic acid extracted by proteinase K and purified by phenol-chloroform and ethanol precipitation was assayed by dot blot hybridization. The probes used were a specific HAV probe corresponding to the 3' end (3D polymerase coding region) and an enterovirus probe corresponding to the 5' noncoding region. The method was first tested under experimental conditions by using virus-spiked shellfish before being applied under field conditions. Our results show that shellfish were highly contaminated: enterovirus and HAV RNAs were found in 63 and 67%, respectively, of samples examined with the riboprobes. On the same site, viral (HAV and enterovirus) RNAs were found in a larger fraction of cockles than mussels. Statistical tests of dependence showed no relationship between viral contamination and bacterial contamination (evaluated by fecal coliform counts).
Full text
PDF

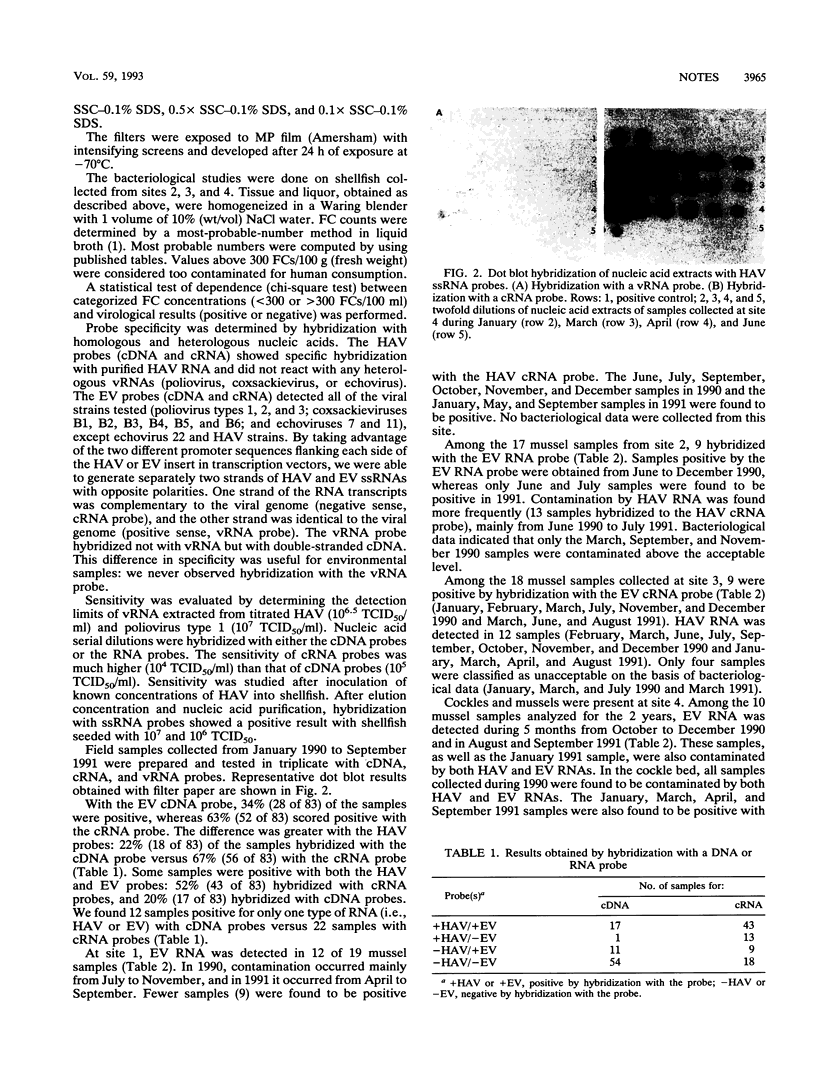
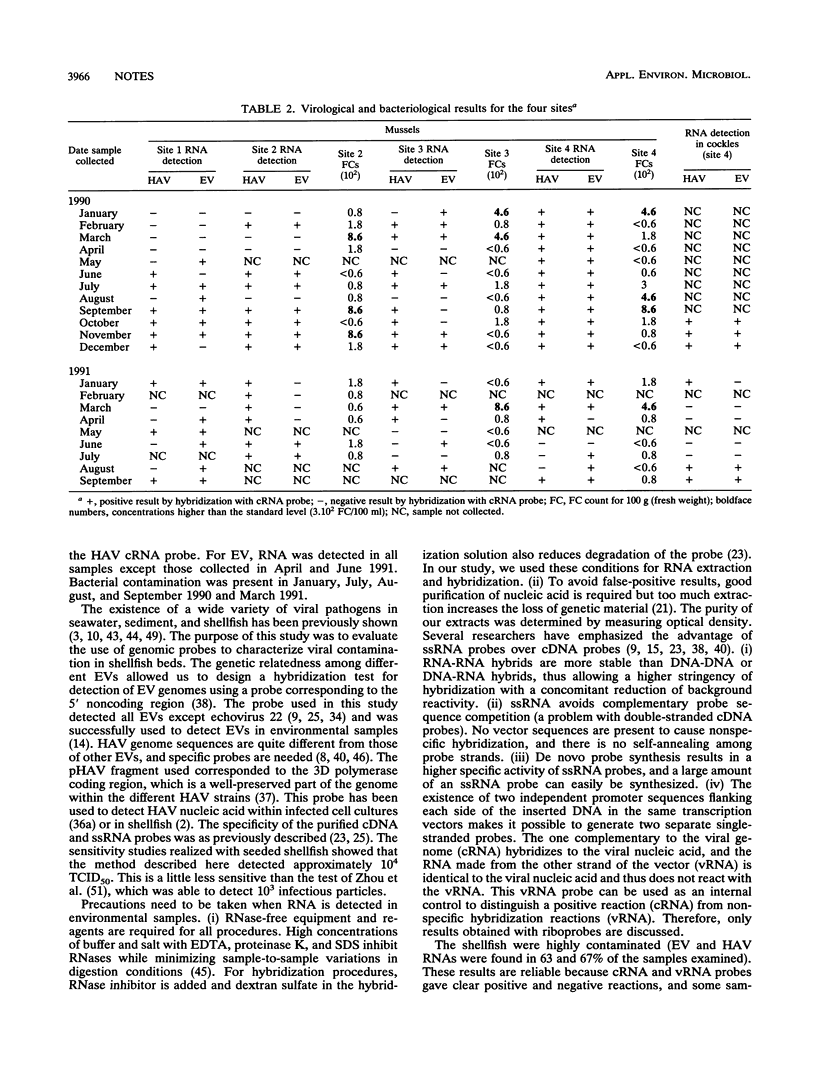


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergh O., Børsheim K. Y., Bratbak G., Heldal M. High abundance of viruses found in aquatic environments. Nature. 1989 Aug 10;340(6233):467–468. doi: 10.1038/340467a0. [DOI] [PubMed] [Google Scholar]
- Bosch A., Lucena F., Girones R., Jofre J. Occurrence of enteroviruses in marine sediment along the coast of Barcelona, Spain. Can J Microbiol. 1988 Jul;34(7):921–924. doi: 10.1139/m88-163. [DOI] [PubMed] [Google Scholar]
- Cohen J. I., Ticehurst J. R., Purcell R. H., Buckler-White A., Baroudy B. M. Complete nucleotide sequence of wild-type hepatitis A virus: comparison with different strains of hepatitis A virus and other picornaviruses. J Virol. 1987 Jan;61(1):50–59. doi: 10.1128/jvi.61.1.50-59.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cova L., Kopecka H., Aymard M., Girard M. Use of cRNA probes for the detection of enteroviruses by molecular hybridization. J Med Virol. 1988 Jan;24(1):11–18. doi: 10.1002/jmv.1890240103. [DOI] [PubMed] [Google Scholar]
- Dahling D. R., Safferman R. S., Wright B. A. Isolation of enterovirus and reovirus from sewage and treated effluents in selected Puerto Rican communities. Appl Environ Microbiol. 1989 Feb;55(2):503–506. doi: 10.1128/aem.55.2.503-506.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desenclos J. C., Klontz K. C., Wilder M. H., Nainan O. V., Margolis H. S., Gunn R. A. A multistate outbreak of hepatitis A caused by the consumption of raw oysters. Am J Public Health. 1991 Oct;81(10):1268–1272. doi: 10.2105/ajph.81.10.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal S. M., Adams W. N., O'Malley M. L., Lear D. W. Human pathogenic viruses at sewage sludge disposal sites in the Middle Atlantic region. Appl Environ Microbiol. 1984 Oct;48(4):758–763. doi: 10.1128/aem.48.4.758-763.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal S. M., Gerba C. P., Melnick J. L. Human enteroviruses in oysters and their overlying waters. Appl Environ Microbiol. 1979 Mar;37(3):572–581. doi: 10.1128/aem.37.3.572-581.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday M. L., Kang L. Y., Zhou T. K., Hu M. D., Pan Q. C., Fu T. Y., Huang Y. S., Hu S. L. An epidemic of hepatitis A attributable to the ingestion of raw clams in Shanghai, China. J Infect Dis. 1991 Nov;164(5):852–859. doi: 10.1093/infdis/164.5.852. [DOI] [PubMed] [Google Scholar]
- Jansen R. W., Newbold J. E., Lemon S. M. Combined immunoaffinity cDNA-RNA hybridization assay for detection of hepatitis A virus in clinical specimens. J Clin Microbiol. 1985 Dec;22(6):984–989. doi: 10.1128/jcm.22.6.984-989.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehl-Pietri C., Dupont J., Herve C., Menard D., Munro J. Occurrence of faecal bacteria, Salmonella and antigens associated with hepatitis A virus in shellfish. Zentralbl Hyg Umweltmed. 1991 Nov;192(3):230–237. [PubMed] [Google Scholar]
- Jiang X., Estes M. K., Metcalf T. G., Melnick J. L. Detection of hepatitis A virus in seeded estuarine samples by hybridization with cDNA probes. Appl Environ Microbiol. 1986 Oct;52(4):711–717. doi: 10.1128/aem.52.4.711-717.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecka H., Prévot J., Girard M., Fuchs F., Aymard M. Intérêt des sondes ARNc (ribosondes) synthétisés in vitro dans la détection des entérovirus par hybridation moléculaire. Ann Inst Pasteur Virol. 1988 Apr-Jun;139(2):217–225. doi: 10.1016/s0769-2617(88)80019-4. [DOI] [PubMed] [Google Scholar]
- Kueh C. S., Grohmann G. S. Recovery of viruses and bacteria in waters off Bondi beach: a pilot study. Med J Aust. 1989 Dec 4;151(11-12):632–638. [PubMed] [Google Scholar]
- Lewis G. D., Metcalf T. G. Polyethylene glycol precipitation for recovery of pathogenic viruses, including hepatitis A virus and human rotavirus, from oyster, water, and sediment samples. Appl Environ Microbiol. 1988 Aug;54(8):1983–1988. doi: 10.1128/aem.54.8.1983-1988.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbithi J. N., Springthorpe V. S., Sattar S. A. Effect of relative humidity and air temperature on survival of hepatitis A virus on environmental surfaces. Appl Environ Microbiol. 1991 May;57(5):1394–1399. doi: 10.1128/aem.57.5.1394-1399.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf T. G., Jiang X. Detection of hepatitis A virus in estuarine samples by gene probe assay. Microbiol Sci. 1988 Oct;5(10):296–300. [PubMed] [Google Scholar]
- Metcalf T. G., Moulton E., Eckerson D. Improved method and test strategy for recovery of enteric viruses from shellfish. Appl Environ Microbiol. 1980 Jan;39(1):141–152. doi: 10.1128/aem.39.1.141-152.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payment P., Tremblay M., Trudel M. Relative resistance to chlorine of poliovirus and coxsackievirus isolates from environmental sources and drinking water. Appl Environ Microbiol. 1985 Apr;49(4):981–983. doi: 10.1128/aem.49.4.981-983.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitjean J., Quibriac M., Freymuth F., Fuchs F., Laconche N., Aymard M., Kopecka H. Specific detection of enteroviruses in clinical samples by molecular hybridization using poliovirus subgenomic riboprobes. J Clin Microbiol. 1990 Feb;28(2):307–311. doi: 10.1128/jcm.28.2.307-311.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao V. C., Metcalf T. G., Melnick J. L. Human viruses in sediments, sludges, and soils. Bull World Health Organ. 1986;64(1):1–13. [PMC free article] [PubMed] [Google Scholar]
- Robertson B. H., Khanna B., Nainan O. V., Margolis H. S. Epidemiologic patterns of wild-type hepatitis A virus determined by genetic variation. J Infect Dis. 1991 Feb;163(2):286–292. doi: 10.1093/infdis/163.2.286. [DOI] [PubMed] [Google Scholar]
- Rotbart H. A. Nucleic acid detection systems for enteroviruses. Clin Microbiol Rev. 1991 Apr;4(2):156–168. doi: 10.1128/cmr.4.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh Y. S., Baric R. S., Sobsey M. D., Ticehurst J., Miele T. A., DeLeon R., Walter R. Detection of hepatitis A virus and other enteroviruses in water by ssRNA probes. J Virol Methods. 1991 Jan;31(1):119–136. doi: 10.1016/0166-0934(91)90150-x. [DOI] [PubMed] [Google Scholar]
- Sobsey M. D., Carrick R. J., Jensen H. R. Improved methods for detecting enteric viruses in oysters. Appl Environ Microbiol. 1978 Jul;36(1):121–128. doi: 10.1128/aem.36.1.121-128.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle C. A., Chan A. M., Cottrell M. T. Use of ultrafiltration to isolate viruses from seawater which are pathogens of marine phytoplankton. Appl Environ Microbiol. 1991 Mar;57(3):721–726. doi: 10.1128/aem.57.3.721-726.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle C. A., Chen F. Mechanisms and rates of decay of marine viruses in seawater. Appl Environ Microbiol. 1992 Nov;58(11):3721–3729. doi: 10.1128/aem.58.11.3721-3729.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticehurst J. R., Feinstone S. M., Chestnut T., Tassopoulos N. C., Popper H., Purcell R. H. Detection of hepatitis A virus by extraction of viral RNA and molecular hybridization. J Clin Microbiol. 1987 Oct;25(10):1822–1829. doi: 10.1128/jcm.25.10.1822-1829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticehurst J. R., Racaniello V. R., Baroudy B. M., Baltimore D., Purcell R. H., Feinstone S. M. Molecular cloning and characterization of hepatitis A virus cDNA. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5885–5889. doi: 10.1073/pnas.80.19.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wommack K. E., Hill R. T., Kessel M., Russek-Cohen E., Colwell R. R. Distribution of viruses in the Chesapeake Bay. Appl Environ Microbiol. 1992 Sep;58(9):2965–2970. doi: 10.1128/aem.58.9.2965-2970.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Sakae K., Ishihara Y., Isomura S. A 2-year survey of the prevalence of enteric viral infections in children compared with contamination in locally-harvested oysters. Epidemiol Infect. 1992 Feb;108(1):155–163. doi: 10.1017/s0950268800049608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. J., Estes M. K., Jiang X., Metcalf T. G. Concentration and detection of hepatitis A virus and rotavirus from shellfish by hybridization tests. Appl Environ Microbiol. 1991 Oct;57(10):2963–2968. doi: 10.1128/aem.57.10.2963-2968.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mesquita M. M., Evison L. M., West P. A. Removal of faecal indicator bacteria and bacteriophages from the common mussel (Mytilus edulis) under artificial depuration conditions. J Appl Bacteriol. 1991 Jun;70(6):495–501. doi: 10.1111/j.1365-2672.1991.tb02746.x. [DOI] [PubMed] [Google Scholar]



