SUMMARY
Schistosomes appear to have evolved several strategies to down-regulate the host's immune response in order to promote their own survival. For the host, down-regulation is also beneficial as it can limit the extent of pathology. It is widely accepted that schistosomes modulate the immune response during the chronic phase of infection after egg deposition has started. However, there is increasing evidence that modulation of the immune response can occur much earlier at the time infective cercariae penetrate the host skin. In this review, we explore the various lines of evidence that excretory/secretory (ES) molecules from cercariae down-regulate the host's immune response. We highlight the immunological factors that are produced and may be involved in regulating the immune system (e.g. IL-10, and eicosanoids), as well as speculating on possible mechanisms of immune modulation (e.g. mast-cell activation, T-cell apoptosis, and/or the skewed activation of antigen-presenting cells [APCs]). Finally, we draw attention to several molecules of schistosome origin that have the potential to stimulate the regulatory response (e.g. glycans) and link these to potential host receptors (e.g. TLRs and C-type lectins).
Keywords: cytokine, helminth, immunomodulation, schistosomes
BACKGROUND
Like many helminth parasites, schistosomes are long lived and can survive in the vertebrate host without causing severe manifestations of disease. It has been estimated that adult schistosome worms can remain in the human host for up to 40 years and each adult worm pair can produce 300 (Schistosoma mansoni) to –3500 (Schistosoma japonicum) eggs per day (1), representing a significant antigenic insult to the host's immune system. The main manifestation of schistosomiasis is the granulomatous response to eggs trapped in host tissues such as the liver, intestines or bladder wall. However, pathology is largely chronic in nature with less than 5% of infected individuals suffering severe disease (2). Therefore, it appears that the host–parasite interaction has evolved to allow continued transmission of the parasite but also to limit severe pathology that may result in the death of the host.
Many studies clearly demonstrate that the chronic phase of infection is characterized by a state of immune hyporesponsiveness exhibited as a reduced ability of host cells to proliferate and a reduction in the size of the granulomatous lesions around embolized ova (2-4). Several excellent articles have recently focused on reviewing the evidence for immune modulation induced by the egg and discuss the different possible mechanisms of action (5-7). Notwithstanding egg-induced immune modulation, there are an increasing number of reports showing that immune regulation begins before egg deposition and that schistosome larvae from the point of infection in the skin are the first stage of the parasite to interfere with the host's immune-defence mechanisms. In this review, we examine the available evidence on the immunoregulatory nature of antigens released from infective larvae as they penetrate the host. This information shows that the released antigens not only stimulate innate immune cells but are also effective at limiting various host immune responses. We draw attention to a number of possible strategies and speculate on the mechanisms that could be used to effect modulation of the immune response, thereby improving the chances of survival of the invading parasite.
Modulation of the host response in vivo by cercariae and skin-stage larvae
Infective schistosome cercariae gain entry to the mammalian or avian host via a percutaneous route and use a number of proteolytic enzymes to digest a route through the skin prior to their exit via blood capillaries or lymphatic vessels (8,9). Ordinarily, schistosomes traverse the skin of their primary host within days, and the vast majority enter the circulation to reach their final site of parasitization. A schematic diagram of schistosome migration through the skin is shown in Figure 1. The precise amount of time larvae spend in the skin has recently been debated (9-11), although evidence from tracking studies of S. mansoni, using both rodent (12) and primate (13) models, show that migration out of the skin can occur within 48–72 h, with the epidermal basement membrane providing the major obstacle for larvae to negotiate. The rate of Schistosoma haematobium migration resembles that of S. mansoni, although larvae of S. japonicum are faster to reach the dermis; in all cases the majority successfully leave the skin (14,15). In contrast, only a minority of bird schistosome Trichobilharzia larvae leave the skin site of infection of either the natural avian (16) or the mammalian host (17). Moreover, evidence from mice repeatedly exposed to Trichobilharzia regenti shows that migration of larvae from the skin is more compromised compared to a single exposure (17), and this occurs via an immune-dependent mechanism (18).
Figure 1.
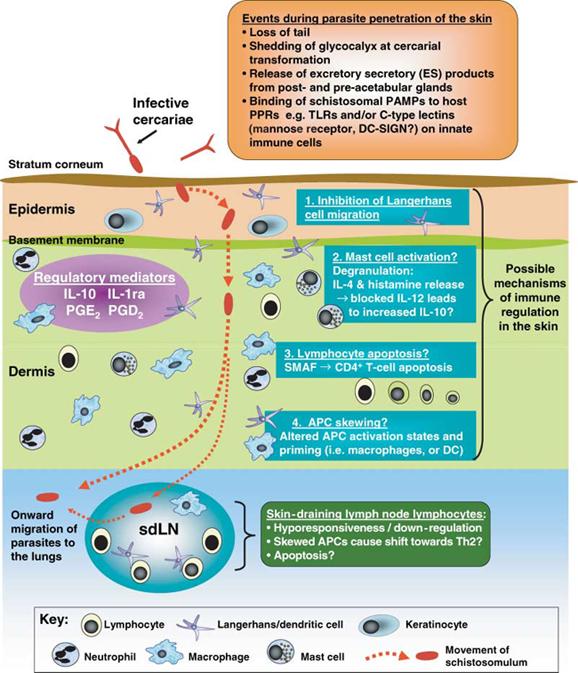
A schematic diagram showing the generalized pattern of schistosome migration through the skin, and the possible mechanisms of immunoregulation that might operate in this tissue. Full details of the regulatory mediators released by both the parasite and the host are given in the main text of the review, as are descriptions of the possible mechanisms of immune regulation and identities of probable ligands. The diagram encompasses data obtained from studies of different Schistosoma and Trichobilarzia species with specific references given in the main text.
While exposure to a small number of cercariae may result in no more than a localized brief inflammation of the skin (19), repeated exposure to S. mansoni (A.P.M.; unpublished data) or T. regenti (17), causes marked cercarial dermatitis. Recent investigations of the inflammatory response in the skin using murine models of exposure to normal and radiation-attenuated (RA) S. mansoni larvae (19-22), or T. regenti (17), reveal that the induction of a heterogeneous influx of polymorphonuclear and mononuclear immune cells in the skin is orchestrated by a localized production of pro-inflammatory cytokines (e.g. IL-1β, IL-12, TNFα, MIP1α and IL-6), as well as mediators with an immunoregulatory function (e.g. IL-10, prostaglandins [PG] E2 and D2) (see Figure 1). Interestingly, a comparison of the three main species of Schistosoma that cause disease in humans shows that while S. japonicum induces a range of pro-inflammatory mediators, S. mansoni and S. haematobium appear only to elicit transcripts for IL-1 receptor antagonist (IL-1ra), IL-10 and TNFα (14). This suggests that there are differences in the induction of pro-inflammatory compared to regulatory mediators, with species that migrate more slowly through the skin favouring the induction of inhibitory IL-1ra and IL-10. Combined, all of the above studies indicate that an innate inflammatory immune response occurs in the skin within hours of cercarial penetration but there is also evidence for the early production of immune regulatory mediators.
Induced immune mediators with regulatory activity
Chief among soluble mediators with known immunoregulatory function and found to be induced early after exposure to schistosome cercariae is IL-10 (14,20,21,23 and see Figure 1). This cytokine has wide-ranging regulatory effects upon antigen presentation, costimulation and the development of acquired T-cell responses (24). Interestingly, RA cercariae, which induce high levels of protective immunity in mice, either fail to induce IL-10 production (19) or elicit a delayed response relative to normal larvae (21). The secretion of IL-10 also occurs later than that of other pro-inflammatory cytokines (e.g. IL-12 and IL-1β), suggesting that it acts to limit their effects (21). This observation is supported by studies using IL-10-deficient mice that have an enhanced inflammatory response in the skin (19,22), coincident with elevated expression within the skin of pro-inflammatory cytokines and MHC II and CD86 (22). These mice also have enhanced Th1-type responses (22,25) and greater levels of immunity when exposed to multiple doses of RA cercariae (25).
It appears that IL-10 is one of the key regulatory mediators of immune responsiveness in skin, especially after exposure to multiple doses of cercariae. We noted that IL-10 production by cells in murine skin was highly elevated after multiple exposures to T. regenti (17) cercariae. The immediate production of IL-10 within 1–3 h of re-exposure suggests it exists in a preformed state but, as yet, a cellular source in the skin has not been identified. Ramaswamy et al. (19) report that keratinocytes are an important source of IL-10, but other potential sources include macrophages, dendritic cells (DCs) or even B1 lymphocytes. Given the recent interest in ‘natural’ CD25+CD4+ regulatory cells (26) and ‘induced’ regulatory CD4+ cells (27), these too are a likely source of IL-10 in the skin, particularly since the number of CD4+ cells increases with time after schistosome infection and after multiple exposure to T. regenti (17) or S. mansoni (A.P.M.; unpublished data). Indeed, CD4+CD25+ cells have been identified as an important feature of the skin of mice that harbour a persistent Leishmania major infection (28). The predicted presence of regulatory T cell involvement, even at this early stage, raises the possibility that TGFβ might also have a modulatory role since production of this cytokine is a hallmark of some T regulatory cells (29). However, at present there is no evidence for, or against, its involvement during early skin phases of parasite migration.
Another group of inhibitory molecules produced after exposure to schistosome larvae are eicosanoids of parasite and/or host origin (19,20,30,31 and see Figure 1). Prostaglandin E2 (PGE2) can function via a cyclooxygenase 2-dependent pathway to aid the production of IL-10 (32) and has been proposed as the prime mediator of schistosome-induced immunoregulation in the mouse skin (19). However, others instead highlight a role for schistosome-derived PGD2 as a factor involved in the IL-10-independent inhibition of host Langerhans cell (LC) migration (20). The inhibitory function of PGD2 is mediated through the membrane-bound prostanoid receptor 1 (DP1), which is expressed on LCs. The migration of LCs as antigen-presenting cells (APC) from the site of schistosome exposure to the draining lymphoid tissue is likely to be important in the subsequent priming of antigen-specific CD4+ responses. Therefore, interruption of the movement of these cells represents a special strategy of the parasite to modulate the host's immune response.
Although various different eicosanoids probably have roles in regulating dermal inflammation, several other mediators may play supporting roles in securing the dominance of immune regulation in the skin. One molecule long known to inhibit the pro-inflammatory functions of IL-1 is IL-1ra. It is produced by human keratinocytes in response to excretory/secretory (ES) products of schistosome larvae (33), although infection of the murine skin does not increase the expression of its transcript (20). It is even possible that an abundance of IL-12p40, rather than acting in concert with p35 as a pro-inflammatory p70 heterodimer (or with p19 as IL-23), may be present in excess as the inhibitory p40 homodimer (34). Together, the above studies show that there are multiple mediators of immune regulation present at the site of parasite exposure. It is likely that all play a role in modulating the host's immune reaction to the invading parasite, possibly as part of a highly coordinated mechanism that has yet to be fully dissected.
Molecules of larval origin with immunomodulatory function
Evidence that schistosome larvae can induce a state of immune regulation in the skin raises the question as to the identity of the parasite molecules responsible. Invasive cercariae and schistosomula in the skin provide the source of stimulatory and inhibitory molecules, particularly those derived from the glycocalyx and released as proteases to aid skin penetration by the parasite (35). ES products of schistosome cercariae, specifically those released by the transforming larvae, are rich in components of the glycocalyx and secretions from the pre- and post-acetabular glands. Most of the glycocalyx is lost by cercariae at infection (35,37), but some of its components remain associated with schistsomula for a period of time after transformation (15,37). The acetabular glands are the source of serine proteases with elastase-like activity, which degrade macromolecules in the skin, thus aiding penetration and migration (9,38); further proteases released from the head gland of skin-stage schistosomula may be responsible for penetration of the basement membrane and the underlying dermis (10).
The immunomodulatory effects of ES products of various helminths has long been realized (39), although relatively few reports have focused on the modulatory effects of ES products released by schistosome cercariae, or the newly transformed larvae. Nevertheless, Vieira et al. (40) were one of the first to report that material released from cercariae could inhibit the in vitro proliferation of human peripheral blood mononuclear cells (PBMCs) from schistosome-infected patients in response to adult worm antigens or to mitogen. The released cercarial preparations also inhibited the response of naïve PBMCs to mitogen. Moreover, when antigen preparations of equal protein concentration from different stages of larval development were compared, it was found that ES products released within the first 3 h of cercarial transformation (termed 0–3 hRP) was less stimulatory (in terms of lymph node cell proliferation) than soluble preparations of whole larvae (41).
Several studies have shown that cercarial ES products when used to immunize naïve skin lead to an oedema and influx of neutrophils (42,43) but it was unclear whether this inflammation was beneficial to the host or the parasite. In fact, although clear evidence for the induction of inflammation was apparent, it was also noted that survival of a secondary infection with cercariae, over the same skin site, was significantly enhanced and this was related to the proteolytic activity of the ES products (43). The same enhancement of adult worm burden was observed following intradermal injection of cercarial ‘glycocalyx’ (44). Combined, these observations could be interpreted to suggest that products of cercariae (proteases and/or glycocalyx) have the ability to modulate the immune response and promote parasite survival, rather than induce host-protective immune mechanisms.
In future studies, it will be important to consider immune regulation at the level of whole tissue (i.e. the skin site of infection) and in relation the rest of the immune circulatory system. However, at this point, progress towards identifying putative parasite ligands of immune regulation have tended to focus upon individual cell types, as will now be discussed.
Activation of mast cells by larval antigens?
The functional role of the Schistosoma ES preparation termed 0–3 hRP released by transforming larvae has been explored using a number of different cell types that might be expected to occur naturally in the skin. For example, ES products are a potent inducer of in vitro mast-cell degranulation and the production of IL-4, histamine and 5-hydroxytryptamine in an IgE-independent manner (45). This mirrors some of the soluble mediators released by the skin after T. regenti infection (17) and supports earlier studies reporting the release from S. mansoni cercariae of a mast cell-triggering factor (46). Although the active molecule(s) has not yet been identified, the ability of schistosome 0–3 hRP to stimulate mast cells is thought to be dependent upon the presence of proteases (45) that are widely known to be important allergens. One candidate molecule might be the schistosome homologue of the human translationally controlled tumour protein (TCTP), which can activate basophils/mast cells (47). Although histamine is generally thought to help activate APCs via increased cytokine production and costimulatory molecule expression (48,49), binding of the H2 histamine receptor inhibits IL-12 (50) thus presenting one way by which activated histamine-producing mast cells regulate the immune response, through the induction of IL-10 (51).
Lymphocyte apoptosis?
Another strategy used by a number of parasites to regulate the host's immune response is parasite-induced apoptosis of host cells (52). One can appreciate the advantage of such a strategy in that the helminth parasite could induce the death of potentially ‘dangerous’ host immune cells and thus subvert the host's attempt to kill the worm or develop a protective acquired response. In schistosomes, a molecule termed ‘S. mansoni apoptosis factor’ (SMAF: rMW 23 kDa) has been identified, which induces apoptosis specifically in the CD4+ lymphocyte population via a Fas–FasL interaction (53). This molecule was found to be a significant component of cercarial ES products and substantially reduced the viability of cells from the skin-draining lymph node during in vitro coculture; in vivo analysis also demonstrated apoptotic cells surrounding invading larvae in the skin (53). These authors suggest that apoptosis of CD4+ cells modulates the host immune responses to allow larvae to escape detection and may be partly mediated by IL-10. As such, it would be interesting to assess the effect of SMAF on regulatory CD4+ cells. Moreover, these authors report that RA larvae do not express this molecule, providing one explanation for why RA larvae are more efficient at stimulating host protective immunity than normal ones.
Skewing the activation of APCs?
Recent studies have focused upon the immune activating and regulatory functions of cercarial ES with respect to potential APCs. Activation of APCs that prime for particular Th phenotypes that are beneficial to the parasite may be a subtle form of immune regulation, and in the context of a single exposure to RA S. mansoni larvae, there is a consensus in the murine model that Th1 cells can mediate an antiparasite host protective response against larval stages (8,54). Recently, we reported how 0–3 hRP activates bone marrow-derived DC by up-regulating limited expression of MHCII, CD40 and CD86, and inducing the release of pro-inflammatory IL-6 and IL-12p40 but not regulatory IL-10 (55). Moreover, DC activated with 0–3 hRP prime CD4+ cells to secrete abundant IL-4, IL-5 and IL-10 in vitro and in vivo indicative of a strong Th2-type phenotype (55). As such, DCs primed with 0–3 hRP resemble those stimulated with SEA (schistosome soluble egg antigen (56,57)) because both drive dominant Th2-type responses. The tendency of DC stimulated with helminth products to promote Th2 responses may reflect their inability to produce Th1-promoting cytokines like IL-12, IL-23 and IL-27 (56). In this respect, neither 0–3 hRP nor SEA induces abundant IL-12p70, unlike ‘Th1-inducing’ LPS, and Propionibacterium acnes (55,56). However, it seems unlikely that the DC is simply not activated by 0–3 hRP, but rather a ‘modified’ maturation state is induced with the limited up-regulation of certain cytokines, MHC II and costimulatory molecules (i.e. CD40 and CD86, but not CD80 or OX40L) (55). We are currently performing proteomic analyses of DCs activated with 0–3 hRP to define the ‘modified’ phenotype and identify ‘hallmarks’ of DC primed with 0–3 hRP in comparison with other Th1-and Th2-activating agents (Ferret-Bernard and Mountford, unpublished data).
The role of CD40–CD154 interaction appears critical to the function of DCs to promote Th2-type responses, as shown by studies using DCs stimulated with Th2-inducing SEA (56,80). We show that the Th2-type responses induced by DCs primed with 0–3 hRP are dramatically altered to a Th1 phenotype by the ligation of CD40 using anti-CD40 antibodies (55). This reversal of phenotype is concurrent with a substantial increase in the secretion of IL-12. However, we have also observed that IL-12 production by skin biopsies within the first few days of parasite exposure are not completely ablated in CD154−/− mice (Hewitson, Hamblin and Mountford, in prep.). This indicates there are different routes leading to the release of Th1-inducing cytokines, and therefore by analogy there are probably multiple pathways by which cercarial ES products skew the phenotype of DCs such that they promote Th2-type responses.
The ‘modified’ DC, or APC, may exist in a state of pathogen-induced suppression to other unrelated microbial products (56). This is observed in DC to occur in response to SEA (58), and we found that optimal doses of 0–3 hRP inhibits IL-12p40 cytokine production by macrophages stimulated with the microbial products LPS or Zymosan A (S. J. Jenkins, unpublished data). This supports an immunomodulatory role for molecules within cercarial ES products. However, in the same experiment, levels of IL-6 and IL-10 were increased, and in studies on DCs, 0–3 hRP increased the secretion of IL-12 in response to CD154-transfected fibroblasts (55). This indicates that 0–3 hRP has differential effects on DCs and macrophages, perhaps as a result of the expression of different surface innate immune receptors (see succeeding discussions). One explanation, as proposed for SEA (58), is that the intracellular fate of the schistosome molecules differs from other pathogen molecules, or even between molecules that comprise the heterogenous mixture of 0–3 hRP. This raises the question as to the identity of host receptor(s) that bind one, or more, of the molecules that comprise 0–3 hRP.
Host receptors involved in recognition of cercarial ES products
Studies using thioglycollate-elicited peritoneal macrophages activated with 0–3 hRP demonstrate that cytokine production (IL-6, IL-12p40) is entirely MyD88-dependent indicating that activation of host cells by 0–3 hRP occurs via a toll-like receptor (TLR)-dependent mechanism (81). In contrast, this study also shows that although IL-12p40 and IL-10 production was largely dependent upon the presence of functional TLR4 (using C3H/HeJ and TLR4−/− mice), the production of IL-6 was largely TLR4 independent, suggesting that different receptors are involved. In vivo studies, however, show that IL-12p40 production in the skin in response to invading larvae is not entirely dependent upon functional TLR4 (21). A separate role for TLR2 ligands within 0–3 hRP has not yet been established, although lipids from schistosome eggs are TLR2 ligands and drive DCs to produce IL-10 (59). The differential response in cytokine production between mice deficient in TLR4 further supports the likelihood that 0–3 hRP is comprised of different molecules with contrasting receptor specificities on host innate immune cells and on APCs in particular.
The involvement of TLR4 would initially suggest the activation of pro-inflammatory cytokine production as occurs following ligation with bacterial products like LPS (60). However, a recent report has identified dextran-conjugated lacto-N-fucopentaose III (LNFPIII) as a glycoconjugate that can ligate TLR4 but suppresses IL-12 production by DCs (61). LNFPIII is a pentasaccharide that terminates in LewisX, which in turn is a trisaccharide that occurs on glycoproteins and glycolipids of schistosomes. DCs activated with LNFPIII prime DCs, which drive Th2-type responses (61) albeit in a MyD88-independent manner (62). However, DCs activated with lacto-neotetraose (LNnT), a tetrasaccharide that does not have α1,3-linked fucose found in LNFPIII, do not drive Th2-type responses (61). It appears that multivalent LewisX/LNFPIII has a strong propensity to modulate the immune response through the induction of IL-10 by PBMC and B-1 cells (63,64). Immunomodulatory molecules that express LewisX, as well as various other fucose-containing glycan elements (e.g. core α1,3-linked fucose and difucosylated LDN), have been isolated from the schistosome egg (62,65-67). LewisX is also present at other different life cycle stages, including cercariae, as are LDN, LDN-F and many other immunogenic glycans (68-72). This raises the possibility that glycans expressed by cercariae or skin-stage larvae also have immunomodulatory activity.
Glycans in the cercarial ES products could bind cell surface C-type lectins such as DC-specific ICAM-3-grabbing non-integrin (DC-SIGN), or the mannose receptor (MR), which in turn modifies the activation of TLRs (73). Both of these C-type lectins are conventionally associated with endocytosis of pathogen-associated material, whereas ligation of TLRs leads to APC activation and the initiation of gene (e.g. cytokine) transcription. Binding of pathogen glycans expressing mannose, fucose or LewisX to DC-SIGN on immature DCs can lead to the induction of tolerance (74,75), and interestingly, DC-SIGN binds to schistosome egg antigens (75,76). The binding of ligands to the MR is reported to stimulate IL-10 and IL-1ra release by DCs that inhibit the induction of Th1 responsiveness, and instead favour the amplification of Th2 circuits (77). In this context, an Fc chimeric protein of the MR (78) has identified an abundance of MR ligands in the glycocaylx compartment of 0–3 hRP that is stimulatory of IL-10 production by macrophages (81, and S. J. Jenkins, unpublished data).
Finally, as an intriguing endnote, researchers have now identified the parasite-derived factor responsible for the observed reduced migration of LCs after exposure to normal cercariae (20). This has been revealed to be the schistosome vaccine candidate glutathione S-transferase that has PGD2 synthase activity (79). This is a further illustration of how the parasite can use its own molecules to promote immune regulation at the earliest point after infection but also shows that an individual molecule can have contrasting effects on the host's immune response.
CONCLUSIONS
Compared to studies on immune regulating activities promoted by the schistosome egg, less is known about the modulatory activities of cercarial ES products. In the above treatise, we summarize the current state of knowledge and describe how immune modulation appears to be operational well before egg deposition occurs. The immunomodulatory actions of invading cercariae are concentrated among those molecules released by the larvae as it penetrates the host skin (e.g. among the glycocalyx and acetabular secretions). The ultimate goal of such activity appears to be the down-regulation of host protective immune responses, and therefore enhance the ability of schistosome larvae to exit the skin en route to their final sites of maturation. Central roles for pro-inflammatory IL-12 and regulatory IL-10 underpin the immune reaction, but other molecules (i.e. eicosanoids) undoubtedly have supporting roles through specific mechanisms (e.g. inhibition of LC migration or of lymphocyte apoptosis). We conclude that the ES products of schistosome cercariae must contain a number of different ligands that can each activate or modulate the host's innate immune system through coordination, or cross talk, between the binding of different TLRs and C-type lectins. Although the biochemical characterization of the parasite ligands of such receptors is at an early stage, it is likely that there will be multiple candidates that will allow for a complex but highly evolved strategy to promote parasite survival in the primary host.
ACKNOWLEDGEMENTS
A.P.M. and G.R.J. were supported by the Wellcome Trust (grants # 071762 and 072255). S.J.J. and J.P.H. were supported by PhD studentships from the Biotechnology and Biological Sciences Research Council. J.P.H. received BBSRC-CASE support from GlaxoSmithKline, Stevenage, Herts U.K. We would like to thank Dr Paul Hamblin at GSK for his supervisory support to JPH, Dr Cornelis Hokke (Department Parasitology, Center of infectious Diseases, Leiden University Medical Center, Leiden, the Netherlands) for helpful discussions on the preparation of the manuscript, the staff of the University of York Biology Service Facility, and Ann Bamford for maintenance of the parasite life cycle.
Abbreviations
- 0–3 hRP
products released by larvae 0–3 h after transformation
- APC
antigen-presenting cell
- DC
dendritic cell
- DC-SIGN
DC-specific ICAM-3-grabbing nonintegrin
- ES
excretory/secretory
- LC
Langerhans cell
- MR
mannose receptor
- PBMC
peripheral blood mononuclear cell
- PG
prostaglandin
- RA
radiation-attenuated
- TLR
toll-like receptor
REFERENCES
- 1.Muller R. Worms and Human Disease. 2nd edn. Wallingford, Oxon, UK: CABI Publishing; 2002. [Google Scholar]
- 2.King CL. Initiation and regulation of disease in schistosomiasis. In: Mahmoud AAF, editor. Schistosomiasis. London: Imperial College Press; 2001. pp. 213–264. Chapter 6. [Google Scholar]
- 3.Stavitsky AB. Regulation of granulomatous inflammation in experimental models of schistosomiasis. Infect Immun. 2004;72:1–12. doi: 10.1128/IAI.72.1.1-12.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinstock JV, Boros DL. Heterogeneity of the granulomatous response in the liver, colon, ileum, and ileal Peyer's patches to schistosome eggs in murine schistosomiasis mansoni. J Immunol. 1981;127:1906–1909. [PubMed] [Google Scholar]
- 5.Stadecker MJ, Asahi H, Finger E, Hernandez HJ, Rutitzky LI, Sun J. The immunobiology of Th1 polarization in high-pathology schistosomiasis. Immunol Rev. 2004;201:168–179. doi: 10.1111/j.0105-2896.2004.00197.x. [DOI] [PubMed] [Google Scholar]
- 6.Wynn TA, Thompson RW, Cheever AW, Mentink-Kane MM. Immunopathogenesis of schistosomiasis. Immunol Rev. 2004;201:156–167. doi: 10.1111/j.0105-2896.2004.00176.x. [DOI] [PubMed] [Google Scholar]
- 7.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 8.Mountford AP, Trottein F. Schistosomes in the skin: a balance between immune priming and regulation. Trends Parasitol. 2004;20:221–226. doi: 10.1016/j.pt.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 9.McKerrow JH, Salter J. Invasion of skin by Schistosoma cercariae. Trends Parasitol. 2002;18:193–195. doi: 10.1016/s1471-4922(02)02309-7. [DOI] [PubMed] [Google Scholar]
- 10.Curwen RS, Wilson RA. Invasion of skin by schistosome cercariae: some neglected facts. Trends Parasitol. 2003;19:63–65. doi: 10.1016/s1471-4922(02)00019-3. [DOI] [PubMed] [Google Scholar]
- 11.Whitfield PJ, Bartlett A, Brown MB, Marriott C. Invasion by schistosome cercariae: studies with human skin explants. Trends Parasitol. 2003;19:339–340. doi: 10.1016/s1471-4922(03)00143-0. [DOI] [PubMed] [Google Scholar]
- 12.Mountford AP, Coulson PS, Wilson RA. Antigen localization and the induction of resistance in mice vaccinated with irradiated cercariae of Schistosoma mansoni. Parasitology. 1988;97(Pt 1):11–25. doi: 10.1017/s0031182000066701. [DOI] [PubMed] [Google Scholar]
- 13.Wilson RA, Coulson PS, Sturrock RF, Reid GD. Schistosome migration in primates: a study in the olive baboon (Papio anubis) Trans R Soc Trop Med Hyg. 1990;84:80–83. doi: 10.1016/0035-9203(90)90391-q. [DOI] [PubMed] [Google Scholar]
- 14.He YX, Chen L, Ramaswamy K. Schistosoma mansoni, S. haematobium, and S. japonicum: early events associated with penetration and migration of schistosomula through human skin. Exp Parasitol. 2002;102:99–108. doi: 10.1016/s0014-4894(03)00024-9. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Li YL, Fishelson Z, Kusel JR, Ruppel A. Schistosoma japonicum migration through mouse skin compared histologically and immunologically with S. mansoni. Parasitol Res. 2005;95:218–223. doi: 10.1007/s00436-004-1284-4. [DOI] [PubMed] [Google Scholar]
- 16.Haas W, Pietsch U. Migration of Trichobilharzia ocellata schistosomula in the duck and the abnormal murine host. Parasitol Res. 1991;77:642–644. doi: 10.1007/BF00931029. [DOI] [PubMed] [Google Scholar]
- 17.Kourilova P, Hogg KG, Kolarova L, Mountford AP. Cercarial dermatitis caused by bird schistosomes comprises both immediate and late phase cutaneous hypersensitivity reactions. J Immunol. 2004;172:3766–3774. doi: 10.4049/jimmunol.172.6.3766. [DOI] [PubMed] [Google Scholar]
- 18.Kourilova P, Syrucek M, Kolarova L. The severity of mouse pathologies caused by the bird schistosome Trichobilharzia regenti in relation to host immune status. Parasitol Res. 2004;93:8–16. doi: 10.1007/s00436-004-1079-7. [DOI] [PubMed] [Google Scholar]
- 19.Ramaswamy K, Kumar P, He YX. A role for parasite-induced PGE2 in IL-10-mediated host immunoregulation by skin stage schistosomula of Schistosoma mansoni. J Immunol. 2000;165:4567–4574. doi: 10.4049/jimmunol.165.8.4567. [DOI] [PubMed] [Google Scholar]
- 20.Angeli V, Faveeuw C, Roye O, et al. Role of the parasite-derived prostaglandin D2 in the inhibition of epidermal Langerhans cell migration during schistosomiasis infection. J Exp Med. 2001;193:1135–1147. doi: 10.1084/jem.193.10.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hogg KG, Kumkate S, Anderson S, Mountford AP. Interleukin-12 p40 secretion by cutaneous CD11c+ and F4/80+ cells is a major feature of the innate immune response in mice that develop Th1-mediated protective immunity to Schistosoma mansoni. Infect Immun. 2003;71:3563–3571. doi: 10.1128/IAI.71.6.3563-3571.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hogg KG, Kumkate S, Mountford AP. IL-10 regulates early IL-12-mediated immune responses induced by the radiation-attenuated schistosome vaccine. Int Immunol. 2003;15:1451–1459. doi: 10.1093/intimm/dxg142. [DOI] [PubMed] [Google Scholar]
- 23.Kumar P, Ramaswamy K. Vaccination with irradiated cercariae of Schistosoma mansoni preferentially induce the accumulation of interferon-gamma producing T cells in the skin and skin-draining lymph nodes of mice. Parasitol Int. 1999;48:109–119. doi: 10.1016/s1383-5769(99)00008-2. [DOI] [PubMed] [Google Scholar]
- 24.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann KF, James SL, Cheever AW, Wynn TA. Studies with double cytokine-deficient mice reveal that highly polarized Th1- and Th2-type cytokine and antibody responses contribute equally to vaccine-induced immunity to Schistosoma mansoni. J Immunol. 1999;163:927–938. [PubMed] [Google Scholar]
- 26.Piccirillo CA, Thornton AM. Cornerstone of peripheral tolerance: naturally occurring CD4+CD25+ regulatory T cells. Trends Immunol. 2004;25:374–380. doi: 10.1016/j.it.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Mills KH, McGuirk P. Antigen-specific regulatory T cells – their induction and role in infection. Semin Immunol. 2004;16:107–117. doi: 10.1016/j.smim.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Mendez S, Reckling SK, Piccirillo CA, Sacks D, Belkaid Y. Role for CD4(+) CD25(+) regulatory T cells in reactivation of persistent leishmaniasis and control of concomitant immunity. J Exp Med. 2004;200:201–210. doi: 10.1084/jem.20040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorelik L, Flavell RA. Transforming growth factor-beta in T-cell biology. Nat Rev Immunol. 2002;2:46–53. doi: 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- 30.Fusco AC, Salafsky B, Delbrook K. Schistosoma mansoni: production of cercarial eicosanoids as correlates of penetration and transformation. J Parasitol. 1986;72:397–404. [PubMed] [Google Scholar]
- 31.Salafsky B, Fusco AC. Schistosoma mansoni: a comparison of secreted vs nonsecreted eicosanoids in developing schistosomulae and adults. Exp Parasitol. 1987;64:361–367. doi: 10.1016/0014-4894(87)90048-8. [DOI] [PubMed] [Google Scholar]
- 32.Harizi H, Juzan M, Pitard V, Moreau JF, Gualde N. Cyclooxygenase-2-issued prostaglandin E2 enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. J Immunol. 2002;168:2255–2263. doi: 10.4049/jimmunol.168.5.2255. [DOI] [PubMed] [Google Scholar]
- 33.Ramaswamy K, Salafsky B, Potluri S, He YX, Li JW, Shibuya T. Secretion of an anti-inflammatory, immunomodulatory factor by Schistosomulae of Schistosoma mansoni. J Inflamm. 1995;46:13–22. [PubMed] [Google Scholar]
- 34.Gately MK, Carvajal DM, Connaughton SE, et al. Interleukin-12 antagonist activity of mouse interleukin-12 p40 homodimer in vitro and in vivo. Ann N Y Acad Sci. 1996;795:1–12. doi: 10.1111/j.1749-6632.1996.tb52650.x. [DOI] [PubMed] [Google Scholar]
- 35.Stirewalt MA. Schistosoma mansoni: cercaria to schistosomule. Adv Parasitol. 1974;12:115–182. doi: 10.1016/s0065-308x(08)60388-7. [DOI] [PubMed] [Google Scholar]
- 36.Cousin CE, Stirewalt MA, Dorsey CH. Schistosoma mansoni: ultrastructure of early transformation of skin- and shear-pressure-derived schistosomules. Exp Parasitol. 1981;51:341–365. doi: 10.1016/0014-4894(81)90122-3. [DOI] [PubMed] [Google Scholar]
- 37.Samuelson JC, Caulfield JP. Loss of covalently labeled glycoproteins and glycolipids from the surface of newly transformed schistosomula of Schistosoma mansoni. J Cell Biol. 1982;94:363–369. doi: 10.1083/jcb.94.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salter JP, Choe Y, Albrecht H, et al. Cercarial elastase is encoded by a functionally conserved gene family across multiple species of schistosomes. J Biol Chem. 2002;277:24618–24624. doi: 10.1074/jbc.M202364200. [DOI] [PubMed] [Google Scholar]
- 39.Lightowlers MW, Rickard MD. Excretory-secretory products of helminth parasites: effects on host immune responses. Parasitology. 1988;96(Suppl):S123–S166. doi: 10.1017/s0031182000086017. [DOI] [PubMed] [Google Scholar]
- 40.Vieira LQ, Gazzinelli G, Kusel JR, De Souza CP, Colley DG. Inhibition of human peripheral blood mononuclear cell proliferative responses by released materials from Schistosoma mansoni cercariae. Parasite Immunol. 1986;8:333–343. doi: 10.1111/j.1365-3024.1986.tb00850.x. [DOI] [PubMed] [Google Scholar]
- 41.Mountford AP, Harrop R, Wilson RA. Antigens derived from lung-stage larvae of Schistosoma mansoni are efficient stimulators of proliferation and gamma interferon secretion by lymphocytes from mice vaccinated with attenuated larvae. Infect Immun. 1995;63:1980–1986. doi: 10.1128/iai.63.5.1980-1986.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teixeira MM, Doenhoff MJ, McNeice C, Williams TJ, Hellewell PG. Mechanisms of the inflammatory response induced by extracts of Schistosoma mansoni larvae in guinea pig skin. J Immunol. 1993;151:5525–5534. [PubMed] [Google Scholar]
- 43.Fallon PG, Teixeira MM, Neice CM, Williams TJ, Hellewell PG, Doenhoff MJ. Enhancement of Schistosoma mansoni infectivity by intradermal injections of larval extracts: a putative role for larval proteases. J Infect Dis. 1996;173:1460–1466. doi: 10.1093/infdis/173.6.1460. [DOI] [PubMed] [Google Scholar]
- 44.Harn DA, Cianci CM, Caulfield JP. Schistosoma mansoni: immunization with cercarial glycocalyx preparation increases the adult worm burden. Exp Parasitol. 1989;68:108–110. doi: 10.1016/0014-4894(89)90015-5. [DOI] [PubMed] [Google Scholar]
- 45.Machado DC, Horton D, Harrop R, Peachell PT, Helm BA. Potential allergens stimulate the release of mediators of the allergic response from cells of mast cell lineage in the absence of sensitization with antigen-specific IgE. Eur J Immunol. 1996;26:2972–2980. doi: 10.1002/eji.1830261224. [DOI] [PubMed] [Google Scholar]
- 46.Catto BA, Lewis FA, Ottesen EA. Cercaria-induced histamine release: a factor in the pathogenesis of schistosome dermatitis? Am J Trop Med Hyg. 1980;29:886–889. doi: 10.4269/ajtmh.1980.29.886. [DOI] [PubMed] [Google Scholar]
- 47.Rao KV, Chen L, Gnanasekar M, Ramaswamy K. Cloning and characterization of a calcium-binding, histamine-releasing protein from Schistosoma mansoni. J Biol Chem. 2002;277:31207–31213. doi: 10.1074/jbc.M204114200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mazzoni A, Young HA, Spitzer JH, Visintin A, Segal DM. Histamine regulates cytokine production in maturing dendritic cells, resulting in altered T cell polarization. J Clin Invest. 2001;108:1865–1873. doi: 10.1172/JCI13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caron G, Delneste Y, Roelandts E, et al. Histamine polarizes human dendritic cells into Th2 cell-promoting effector dendritic cells. J Immunol. 2001;167:3682–3686. doi: 10.4049/jimmunol.167.7.3682. [DOI] [PubMed] [Google Scholar]
- 50.van der Pouw Kraan TC, Snijders A, Boeije LC, et al. Histamine inhibits the production of interleukin-12 through interaction with H2 receptors. J Clin Invest. 1998;102:1866–1873. doi: 10.1172/JCI3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jutel M, Watanabe T, Akdis M, Blaser K, Akdis CA. Immune regulation by histamine. Curr Opin Immunol. 2002;14:735–740. doi: 10.1016/s0952-7915(02)00395-3. [DOI] [PubMed] [Google Scholar]
- 52.James ER, Green DR. Manipulation of apoptosis in the host–parasite interaction. Trends Parasitol. 2004;20:280–287. doi: 10.1016/j.pt.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 53.Chen L, Rao KV, He YX, Ramaswamy K. Skin-stage schistosomula of Schistosoma mansoni produce an apoptosis-inducing factor that can cause apoptosis of T cells. J Biol Chem. 2002;277:34329–34335. doi: 10.1074/jbc.M201344200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dunne DW, Mountford AP. Resistance to infection in humans and animal models. In: Mahmoud AAF, editor. Schistosomiasis. London: Imperial College Press; 2001. pp. 133–212. Chapter 5. [Google Scholar]
- 55.Jenkins SJ, Mountford AP. Dendritic cells activated with products released by schistosome larvae drive Th2-type immune responses, which can be inhibited by manipulation of CD40 costimulation. Infect Immun. 2005;73:395–402. doi: 10.1128/IAI.73.1.395-402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pearce EJ, Kane CM, Sun J, Taylor JJ, McKee AS, Cervi L. Th2 response polarization during infection with the helminth parasite Schistosoma mansoni. Immunol Rev. 2004;201:117–126. doi: 10.1111/j.0105-2896.2004.00187.x. [DOI] [PubMed] [Google Scholar]
- 57.MacDonald AS, Straw AD, Bauman B, Pearce EJ. CD8–dendritic cell activation status plays an integral role in influencing Th2 response development. J Immunol. 2001;167:1982–1988. doi: 10.4049/jimmunol.167.4.1982. [DOI] [PubMed] [Google Scholar]
- 58.Cervi L, MacDonald AS, Kane C, Dzierszinski F, Pearce EJ. Cutting edge: dendritic cells copulsed with microbial and helminth antigens undergo modified maturation, segregate the antigens to distinct intracellular compartments, and concurrently induce microbe-specific Th1 and helminth-specific Th2 responses. J Immunol. 2004;172:2016–2020. doi: 10.4049/jimmunol.172.4.2016. [DOI] [PubMed] [Google Scholar]
- 59.van der Kleij D, Latz E, Brouwers JF, et al. A novel host–parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates Toll-like receptor 2 and affects immune polarization. J Biol Chem. 2002;277:48122–48129. doi: 10.1074/jbc.M206941200. [DOI] [PubMed] [Google Scholar]
- 60.Underhill DM, Gantner B. Integration of Toll-like receptor and phagocytic signaling for tailored immunity. Microbes Infect. 2004;6:1368–1373. doi: 10.1016/j.micinf.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 61.Thomas PG, Carter MR, Atochina O, et al. Maturation of dendritic cell 2 phenotype by a helminth glycan uses a Toll-like receptor 4-dependent mechanism. J Immunol. 2003;171:5837–5841. doi: 10.4049/jimmunol.171.11.5837. [DOI] [PubMed] [Google Scholar]
- 62.Thomas PG, Harn DA., Jr Immune biasing by helminth glycans. Cell Microbiol. 2004;6:13–22. doi: 10.1046/j.1462-5822.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 63.Velupillai P, Secor WE, Horauf AM, Harn DA. B-1 cell (CD5+B220+) outgrowth in murine schistosomiasis is genetically restricted and is largely due to activation by polylactosamine sugars. J Immunol. 1997;158:338–344. [PubMed] [Google Scholar]
- 64.Velupillai P, dos Reis EA, dos Reis MG, Harn DA. Lewis(x)-containing oligosaccharide attenuates schistosome egg antigen-induced immune depression in human schistosomiasis. Hum Immunol. 2000;61:225–232. doi: 10.1016/s0198-8859(99)00136-6. [DOI] [PubMed] [Google Scholar]
- 65.van der Kleij D, Van Remoortere A, Schuitemaker JH, et al. Triggering of innate immune responses by schistosome egg glycolipids and their carbohydrate epitope GalNAc beta 1–4(Fuc alpha 1–2Fuc alpha 1–3)GlcNAc. J Infect Dis. 2002;185:531–539. doi: 10.1086/338574. [DOI] [PubMed] [Google Scholar]
- 66.Nyame AK, Kawar ZS, Cummings RD. Antigenic glycans in parasitic infections: implications for vaccines and diagnostics. Arch Biochem Biophys. 2004;426:182–200. doi: 10.1016/j.abb.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 67.Faveeuw C, Mallevaey T, Paschinger K, et al. Schistosome N-glycans containing core alpha 3-fucose and core beta 2-xylose epitopes are strong inducers of Th2 responses in mice. Eur J Immunol. 2003;33:1271–1281. doi: 10.1002/eji.200323717. [DOI] [PubMed] [Google Scholar]
- 68.Nyame AK, Lewis FA, Doughty BL, Correa-Oliveira R, Cummings RD. Immunity to schistosomiasis: glycans are potential antigenic targets for immune intervention. Exp Parasitol. 2003;104:1–13. doi: 10.1016/s0014-4894(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 69.Robijn ML, Wuhrer M, Kornelis D, Deelder AM, Geyer R, Hokke CH. Mapping fucosylated epitopes on glycoproteins and glycolipids of Schistosoma mansoni cercariae, adult worms and eggs. Parasitology. 2005;130:67–77. doi: 10.1017/s0031182004006390. [DOI] [PubMed] [Google Scholar]
- 70.Van Remoortere A, Hokke CH, van Dam GJ, van Die I, Deelder AM, van den Eijnden DH. Various stages of Schistosoma express Lewis(x), LacdiNAc, GalNAcbeta1–4(Fucalpha1–3)GlcNAc and GalNAcbeta1–4(Fucalpha1–2Fucalpha1–3) GlcNAc carbohydrate epitopes: detection with monoclonal antibodies that are characterized by enzymatically synthesized neoglycoproteins. Glycobiology. 2000;10:601–609. doi: 10.1093/glycob/10.6.601. [DOI] [PubMed] [Google Scholar]
- 71.Huang HH, Tsai PL, Khoo KH. Selective expression of different fucosylated epitopes on two distinct sets of Schistosoma mansoni cercarial O-glycans: identification of a novel core type and Lewis X structure. Glycobiology. 2001;11:395–406. doi: 10.1093/glycob/11.5.395. [DOI] [PubMed] [Google Scholar]
- 72.Wuhrer M, Dennis RD, Doenhoff MJ, Lochnit G, Geyer R. Schistosoma mansoni cercarial glycolipids are dominated by Lewis X and pseudo-Lewis Y structures. Glycobiology. 2000;10:89–101. doi: 10.1093/glycob/10.1.89. [DOI] [PubMed] [Google Scholar]
- 73.van Kooyk Y, Engering A, Lekkerkerker AN, Ludwig IS, Geijtenbeek TB. Pathogens use carbohydrates to escape immunity induced by dendritic cells. Curr Opin Immunol. 2004;16:488–493. doi: 10.1016/j.coi.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 74.van Kooyk Y, Geijtenbeek TB. DC-SIGN: escape mechanism for pathogens. Nat Rev Immunol. 2003;3:697–709. doi: 10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- 75.Appelmelk BJ, van Die I, van Vliet SJ, Vandenbroucke-Grauls CM, Geijtenbeek TB, van Kooyk Y. Cutting edge: carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J Immunol. 2003;170:1635–1639. doi: 10.4049/jimmunol.170.4.1635. [DOI] [PubMed] [Google Scholar]
- 76.van Die I, van Vliet SJ, Nyame AK, et al. The dendritic cell-specific C-type lectin DC-SIGN is a receptor for Schistosoma mansoni egg antigens and recognizes the glycan antigen Lewis x. Glycobiology. 2003;13:471–478. doi: 10.1093/glycob/cwg052. [DOI] [PubMed] [Google Scholar]
- 77.Chieppa M, Bianchi G, Doni A, et al. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J Immunol. 2003;171:4552–4560. doi: 10.4049/jimmunol.171.9.4552. [DOI] [PubMed] [Google Scholar]
- 78.Martinez-Pomares L, Kosco-Vilbois M, Darley E, et al. Fc chimeric protein containing the cysteine-rich domain of the murine mannose receptor binds to macrophages from splenic marginal zone and lymph node subcapsular sinus and to germinal centers. J Exp Med. 1996;184:1927–1937. doi: 10.1084/jem.184.5.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Herve M, Angeli V, Pinzar E, et al. Pivotal roles of the parasite PGD2 synthase and of the host D prostanoid receptor 1 in schistosome immune evasion. Eur J Immunol. 2003;33:2764–2772. doi: 10.1002/eji.200324143. [DOI] [PubMed] [Google Scholar]
- 80.MacDonald AS, Straw AD, Dalton NM, Pearce EJ. Th2 response induction by dendritic cells: a role for CD40. J Immunol. 2002;168:537–540. doi: 10.4049/jimmunol.168.2.537. [DOI] [PubMed] [Google Scholar]
- 81.Jenkins SJ, Hewitson JP, Ferret-Bernard S, Mountford AP. Schistosome larvae stimulate macrophage cytokine production through TLR4-dependent and independent pathways. Int Immunol. 2005 doi: 10.1093/intimm/dxh319. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


