Abstract
A new class of pyridine oxide derivatives as inhibitors of human immunodeficiency virus type 1 (HIV-1) and/or HIV-2 replication in cell culture has been identified. The compounds, which specifically inhibit HIV-1, behave as typical nonnucleoside reverse transcriptase inhibitors (NNRTIs). The most active congener of this group, JPL-133 (UC-B3096), has a 50% effective concentration of 0.05 μg/ml for HIV-1(IIIB) with a selectivity index of approximately 760 in CEM cell cultures. However, the cytostatic activity of most pyridine oxide derivatives highly depended on the nature of the cell line. All compounds, including those pyridine oxide derivatives that inhibit both HIV-1 and HIV-2 replication, select for NNRTI-characteristic mutations in the HIV-1 reverse transcriptase of HIV-infected cell cultures (i.e., Lys103Asn, Val108Ile, Glu138Lys, Tyr181Cys and Tyr188His). These amino acid mutations emerged mostly through transition of guanine to adenine or adenine to guanine in the corresponding codons of the reverse transcriptase (RT) gene. The HIV-1-specific pyridine oxide derivatives lost their antiviral activity against HIV-1 strains containing these mutations in the RT. However, most compounds retained pronounced antiviral potency against virus strains that contained other NNRTI-characteristic RT mutations, such as Leu100Ile and Val179Asp. Furthermore, the complete lack of inhibitory activity of the pyridine oxide derivatives against recombinant HIV-2 RT and partial retention of anti-HIV-1 activity against HIV-1 strains that contain a variety of HIV-1-characteristic mutations suggest that the pyridine oxide derivatives must have a second target of antiviral action independent from HIV-1 RT.
AIDS, caused by the human immunodeficiency virus (HIV), remains a health threat of global significance. There are currently 16 drugs approved for the treatment of HIV-infected individuals; these drugs consist of six nucleoside reverse transcriptase inhibitors, three nonnucleoside reverse transcriptase inhibitors (NNRTI), one acyclic nucleoside phosphonate reverse transcriptase (RT) inhibitor, and six protease inhibitors (for reviews, see references 4, 7, and 8). The structurally diverse classes of NNRTIs consist of highly potent and selective inhibitors of HIV type 1 (HIV-1) RT that prevent the synthesis and subsequent incorporation of proviral DNA in the cellular genome. A major drawback of this class of anti-HIV compounds, however, is the rapid emergence of drug-resistant virus strains, resulting in cross-resistance to other NNRTIs. X-ray crystallographic structures have shown that HIV-1 resistance to NNRTIs is mediated through mutations of amino acid residues lining a hydrophobic NNRTI-binding pocket, which is located in the palm domain of the p66 subunit of RT (9). The original NNRTIs, such as nevirapine and delavirdine, select for single amino acid mutations that commonly occur at several amino acid positions between codons 98 and 108, 179 and 190, and 225 and 236 of the HIV-1 RT. However, the more recent NNRTIs, such as efavirenz, quinoxalines, and UC-781, require the presence of multiple mutations in the HIV-1 RT for pronounced drug resistance of the mutant virus strains. Because of the limitation of currently available anti-HIV drugs, extensive search for new anti-HIV agents is still necessary and ongoing.
We recently reported the discovery and structure-activity relationship of a group of pyridine oxide derivatives that are endowed with pronounced anti-HIV properties in cell culture (5). It was found that a number of them were very selective in inhibiting HIV-1 but not HIV-2. Other pyridine oxide derivatives, however, were also inhibitory to HIV-2, presumably through another mechanism of action. In this study, the antiviral properties and the resistance pattern of HIV-1 for those pyridine oxide compounds that show selective anti-HIV-1 activity will be reported.
MATERIALS AND METHODS
Compounds.
Pyridine oxide derivatives (designated JPL) (Table 1) were supplied by Crompton Corporation (Middlebury, Conn. and Guelph, Canada). The compounds, containing a substituent other than hydrogen at the Z position, were tested as racemic (R-S) mixtures. UC-781 was obtained from W. Brouwer (Crompton Corporation, Guelph, Canada). Nevirapine (BI-RG-587; dipyridodiazepinone) was provided by P. Ganong (Boehringer Ingelheim, Ridgefield, Conn.), and efavirenz (DMP266) was provided by R. Kirsch (Hoechst AG, Frankfurt, Germany). Tenofovir [PMPA, 9-(2-phosphonylmethoxypropyl)adenine] was a kind gift from N. Bischofberger (Gilead Sciences, Foster City, Calif.). Zidovudine (AZT) was obtained from D. G. Johns (National Institutes of Health, Bethesda, Md.), and lamivudine (3TC) was provided by J. Cameron (Glaxo-Wellcome, Stevenage, United Kingdom).
TABLE 1.
Structural formulae of pyridine oxide derivatives
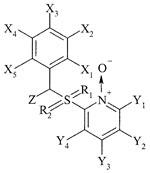 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Element or molecule(s) at position:
|
|||||||||||
| X1 | X2 | X3 | X4 | X5 | Z | R1 | R2 | Y1 | Y2 | Y3 | Y4 | |
| JPL-10 | Me | H | H | Me | H | H | O | O | H | H | H | H |
| JPL-27 | H | Me | H | H | H | H | O | O | H | H | H | H |
| JPL-30 | OMe | H | H | Me | H | H | O | O | H | H | H | H |
| JPL-71 | Me | H | H | Me | H | Me | O | O | H | H | H | Me |
| JPL-74 | Me | H | H | Me | H | Cl | O | O | H | H | Me | H |
| JPL-78 | Me | H | Me | H | H | Me | O | O | H | H | H | Me |
| JPL-107a | H | H | H | H | H | Me | O | O | ||||
| JPL-110 | Me | H | H | Me | H | Me | O | O | H | Me | H | H |
| JPL-133 | Me | H | H | Me | H | H | H | H | H | Cl | ||
| JPL-146 | H | H | H | H | H | H | H | H | H | NO2 | ||
| JPL-150b | Me | H | H | Me | H | H | O | O | H | H | H | H |
The pyridine oxide has been replaced by an 8-ethyl-4-methylquinoline-2-yl moiety.
The pyridine oxide has been replaced by its reduced form.
Cells and viruses.
Human lymphocyte CEM cells were obtained from the American Tissue Cell Culture Collection (Rockville, Md.). MT-4 cells were provided by N. Yamamoto (Tokyo Medical School and Dental University School of Medicine, Tokyo, Japan). C8166 cells were obtained from P. La Colla (Cagliari, Sardinia), and MT-2 cells were obtained from L. Montagnier (Pasteur Institute, Paris, France). Cells were maintained in RPMI-1640 (Life Technologies, Merelbeke, Belgium), supplemented with 10% heat-inactivated fetal calf serum (Integro, Zaandam, The Netherlands), 2 mM l-glutamine (Life Technologies) and 0.1% NaHCO3 (Life Technologies), and incubated at 37°C in a humidified CO2-controlled atmosphere. HIV-1(IIIB), HIV-1(MN), and HIV-1(RF) (11) were provided by R. C. Gallo and M. Popovic (National Institutes of Health, Bethesda, Md.). HIV-1(HE) represents a clinical isolate obtained from a patient with AIDS in Leuven, Belgium. HIV-2(ROD) (6) and HIV-2(EHO) (12) were both provided by L. Montagnier.
Antiviral activity assays.
The procedures for assessing the anti-HIV activity in cell culture have been described previously (2, 3) and are based on the inhibition of HIV-induced giant cell formation in CEM, C8166, and MT-2 cell cultures at day 4 postinfection by microscopic examination. Briefly, cells were suspended at 250,000 cells ml−1 in culture medium and infected with HIV at approximately 100 times the 50% cell culture infectious dose per ml. Then 100 μl of the infected cell suspension was added to a 96-well plate containing 100 μl of serial dilutions of the test compounds. After 4 days of incubation at 37°C, the cell cultures were examined for syncytium formation. The 50% effective concentration (EC50) was defined as the compound concentration required to inhibit virus-induced syncytium formation by 50%. The anti-HIV activity in MT-4 cells was based on cell viability. The cells had been infected with 100 50% cell culture infectious doses of HIV in the presence of various concentrations of the test compounds. After the MT-4 cells had been allowed to proliferate for 5 days at 37°C, the number of viable cells was quantified by the trypan blue exclusion method. The EC50 was defined as the compound concentration required to inhibit virus-induced cell death by 50%.
Selection of HIV-1(IIIB) mutant strains.
HIV-1(IIIB)-infected CEM cells in 1-ml cell cultures (∼1.2 × 105 cells/ml) were subjected to serial passages (i.e., every third or fourth day of cultivation) in the presence of a variety of pyridine oxide derivatives at different fixed concentrations. Virus breakthrough became visible as syncytium formation in the virus-infected CEM cell cultures and was estimated as the percentage of the cell cultures that contained HIV-1-induced syncytia. HIV-1-infected CEM cell cultures that were not exposed to the test compounds served as the control. The number of giant cells that appeared in these HIV-1-infected control cell cultures 4 days postsubcultivation were estimated microscopically and arbitrarily designated as 100%.
Determination of the amino acid sequence of the RT of drug-resistant virus strains.
CEM cells infected with the HIV-1 mutant strains were incubated for 3 days, centrifuged, washed twice with phosphate-buffered saline, and resuspended in 200 μl of phosphate-buffered saline. The cell suspension was subjected to total DNA isolation with the QIAamp DNA blood kit (Qiagen, Leusden, The Netherlands). Amplification of proviral DNA (40 cycles) was performed in 10 mM Tris-HCl (pH 8.8), 50 mM KCl, 1.5 mM MgCl2, 0.1% Triton X-100, 2.5 U of thermostable DNA polymerase (Dyna Zyme, Finnzymes), and 15 μM concentrations of all primers in a final volume of 50 μl. The primers 5′-AATTGTTTTACATCATTAGTGTG and 5′-GGGGTTAAATAAAATAGTAAG gave a 2,060-bp product of the proviral RT gene. A new 30-cycle PCR with a second set of primers (5′-GCTACACTAGAAGAAATGATGAC and 5′-CTTGATAAATTTGATATGTCCATTG) generated a 1,770-bp RT fragment. The PCR products were purified on Microspin S-400 HR columns (Pharmacia, Montreal, Quebec, Canada), sequenced with a Taq dye deoxy terminator sequencing kit (Applied Biosystems), and analyzed with a DNA sequencer (model 373A from Applied Biosystems).
RT assay.
The RT assays using recombinant HIV-1 RT were performed as described previously (1). Briefly, the reaction mixture (50 μl) contained 50 mM Tris-HCl (pH 7.8), 5 mM dithiothreitol, 300 mM glutathione, 500 μM EDTA, 150 mM KCl, 5 mM MgCl2, 1.25 μg of bovine serum albumin, a fixed concentration of the labeled substrate (2 μCi per assay for either [3H]dTTP [0.72 μM], [3H]dCTP [2.1 μM] or [3H]dGTP [13.8 μM]), a fixed concentration of the template-primer [poly(rA · dT) (0.015 mM), poly(rC · dG) (0.1 mM), or poly(rI · dC) (0.015 mM)], 0.06% Triton X-100, 10 μl of inhibitor solution (containing various concentrations of the compounds), and 5 μl of the purified recombinant HIV-1 RT preparation. The reaction mixtures were incubated at 37°C for 60 min, at which time 200 μl of calf thymus DNA in H2O (2 mg ml−1) and 1 ml of saturated sodium phosphate buffer (equimolar amounts of NaH2PO4 and Na2HPO4) in a 5% (vol/vol) aqueous trichloroacetic acid solution were added. The solutions were kept on ice for 30 min, after which the acid-insoluble material was washed and analyzed for radioactivity.
RESULTS
Antiviral activity spectrum of pyridine oxide derivatives.
The anti-HIV activities of a selected number of pyridine oxide derivatives (Table 1) were evaluated in a variety of human lymphocyte cell lines, including CEM, C8166, MT-2, and MT-4 cells (Table 2), against three HIV-1 laboratory strains (IIIB, RF, and MN), a clinical HIV-1 isolate (HE), and two HIV-2 strains (ROD and EHO) (Table 3). All compounds were inhibitory to HIV-1(IIIB) replication in CEM cells at concentrations that were well below their toxicity threshold in the host cells. Similar EC50 values were also found for the pyridine oxides derivatives, irrespective of the nature of the host cell type. In contrast, the cytotoxicity of the compounds showed more variation depending upon the cell line used and was most pronounced in C8166 cell cultures for most of the tested compounds (Table 2). The antiviral activities against HIV-1 strains other than IIIB were quite comparable to each other, not only in CEM cell cultures (Table 3), but also in other cell cultures (C8166 and MT-2) (data not shown). Whereas JPL-10, JPL-27, and JPL-30 also inhibited HIV-2 strains in CEM cell cultures to an almost similar (JPL-10 and JPL-27) or slightly lesser (six to sevenfold) extent (JPL-30) than they inhibited HIV-1(IIIB), the other pyridine oxide derivatives completely lost antiviral activity against the HIV-2 strains.
TABLE 2.
Anti-HIV-1(IIIB) activity of test compounds in CEM, C8166, MT-2, and MT-4 cellsa
| Compound | CEM
|
C8166
|
MT-2
|
MT-4
|
||||
|---|---|---|---|---|---|---|---|---|
| EC50 | CC50 | EC50 | CC50 | EC50 | CC50 | EC50 | CC50 | |
| JPL-10 | 5.3 ± 2.6 | 31 ± 6 | 6.1 ± 1.5 | 9.8 ± 3.2 | 8.0 ± 1.3 | 8.0 ± 3.1 | 7.9 ± 1.7 | 34 ± 1 |
| JPL-27 | 6.8 ± 3.5 | 29 ± 6 | 7.0 ± 4.1 | 29 ± 1 | 16 ± 3 | 26 ± 1 | 17 ± 4 | 30 ± 12 |
| JPL-30 | 5.9 ± 3.0 | 70 ± 20 | 5.7 ± 2.5 | 59 ± 16 | 12 ± 2 | 26 ± 3 | 9.1 ± 3.4 | ≥100 |
| JPL-71 | 0.10 ± 0.01 | 36 ± 3 | 0.10 ± 0.04 | 1.7 ± 0.4 | 0.20 ± 0.10 | 11 ± 1 | 0.12 ± 0.01 | 11 ± 2 |
| JPL-74 | 0.48 ± 0.01 | >4b | 0.40 ± 0.11 | 1.4 ± 0.2 | 0.64 ± 0.23 | >4b | 1.3 ± 0.2 | >4b |
| JPL-78 | 3.4 ± 0.9 | >100 | 5.0 ± 2.9 | 6.9 ± 3.2 | 8.6 ± 2.0 | >100 | 2.4 ± 0.1 | 60 ± 30 |
| JPL-107 | 0.52 ± 0.03 | >4b | 1.0 ± 0.4 | 1.6 ± 0.3 | 1.3 ± 0.5 | >4b | 0.54 ± 0.05 | >4b |
| JPL-110 | 9.6 ± 1.4 | ≥100 | ≥4 | 2.8 ± 0.1 | 23 ± 11 | 34 ± 14 | 11 ± 5 | 26 ± 10 |
| JPL-133 | 0.05 ± 0.01 | 38 ± 6 | 0.04 ± 0.01 | 6.2 ± 0.7 | 0.10 ± 0.03 | 16 ± 7 | 0.12 ± 0.01 | 13 ± 2 |
| JPL-146 | 2.2 ± 0.2 | 30 ± 7 | 2.8 ± 0.8 | 29 ± 10 | 2.4 ± 0.8 | 21 ± 4 | 2.8 ± 0.8 | 28 ± 12 |
| JPL-150 | 4.0 ± 0.1 | 42 ± 10 | 4.0 ± 0.9 | 30 ± 5 | 8.8 ± 2.0 | 28 ± 8 | 8.1 ± 3.2 | 46 ± 7 |
EC50, compound concentration required to inhibit HIV-1-induced cytopathicity (giant cell formation) in CEM, C8166, and MT-2 cells or HIV-induced cytopathicity (cell lysis) in MT-4 cells by 50%; CC50, compound concentration required to reduce cell viability (MT-4) or to inhibit cell proliferation (CEM, C8166, and MT-2) by 50%. Data represent the average values (± standard deviation) for at least three independent experiments.
Higher drug concentrations could not be accurately determined due to insolubility of the compound at these concentrations.
TABLE 3.
Antiviral activity of pyridine oxide derivatives against different HIV strains in CEM cell cultures
| Compound | EC50 (μg/ml)a
|
||||
|---|---|---|---|---|---|
| HIV-1 strain
|
HIV-2 strain
|
||||
| RF | MN | HE | ROD | EHO | |
| JPL-10 | 7.2 ± 2.6 | 10 ± 1 | 11 ± 4 | 10 ± 1 | 14 ± 1 |
| JPL-27 | 10 ± 2 | 13 ± 1 | 10 ± 2 | 12 ± 2 | 14 ± 2 |
| JPL-30 | 10 ± 1 | 40 ± 4 | 38 ± 2 | 40 ± 4 | 44 ± 5 |
| JPL-71 | 0.3 ± 0.1 | 1.4 ± 0.3 | 0.4 ± 0.1 | >20 | >20 |
| JPL-74 | 1.0 ± 0.3 | ≥4b | ≥4b | >4b | >4b |
| JPL-78 | 7.7 ± 3.3 | 36 ± 16 | 17 ± 10 | >100 | >100 |
| JPL-107 | 1.1 ± 0.6 | 2.4 ± 0.2 | 4 ± 1 | >4b | >4b |
| JPL-110 | 48 ± 10 | 60 ± 1 | 40 ± 9 | >100 | >100 |
| JPL-133 | 0.16 ± 0.10 | 0.4 ± 0.1 | 0.2 ± 0.1 | >20 | ≥20 |
| JPL-146 | 8.4 ± 0.5 | 13 ± 1 | 10 ± 2 | ≥20 | >20 |
| JPL-150 | 8.4 ± 0.5 | 20 ± 4 | 14 ± 1 | >20 | >20 |
EC50, compound concentration required to inhibit HIV-1-induced cytopathicity (giant cell formation) in CEM cell cultures. Data represent the average values (± standard deviation) for at least three independent experiments.
Higher drug concentrations could not be accurately determined due to insolubility of the compound at these concentrations.
Anti-HIV RT activity of JPL derivatives.
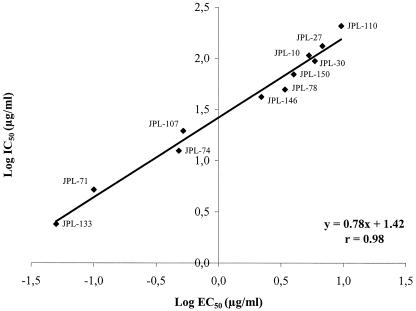
The pyridine oxide derivatives were evaluated for their inhibitory effects against purified recombinant HIV-1 and HIV-2 RT (Table 4). The most active pyridine oxide congeners (JPL-133 and JPL-71) inhibited HIV-1 RT at 50% inhibitory concentrations (IC50s) of 2.4 μg/ml and 5.2 μg/ml, respectively. Also JPL-10 and JPL-30, which were among the least effective HIV-1 inhibitors (EC50, ∼5 μg/ml), inhibited HIV-1 RT at IC50 values of 107 and 94 μg/ml, respectively. For all the pyridine oxide derivatives that had been evaluated for their anti-HIV-1 RT activity [using poly(rC · dG) as the template and dGTP as the radiolabeled substrate], the IC50 values were plotted against their EC50 values for antiviral activity (Fig. 1). A very close correlation between anti-HIV-1 RT activity and antiviral activity in CEM cell cultures was found. The drug concentrations required to inhibit HIV-1 RT were, as a rule, 15- to 50-fold higher than those required to inhibit HIV-1 replication in cell culture. The inhibitory effect on HIV-1 RT in the presence of other artificial homopolymeric templates was also examined (Table 4). The compounds showed comparable inhibitory effects against HIV-1 RT in the presence of poly(rC · dG) (using [3H]dGTP as substrate) and poly(rA · dT) (using [3H]dTTP as substrate) but were, as a rule, ∼50-fold less effective when poly(rI · dC) (using [3H]dCTP as substrate) was used as the template-primer. Interestingly, none of the pyridine oxide derivatives, including JPL-10, JPL-27, and JPL-30, that were active against both HIV-1 and HIV-2 in cell culture were inhibitory to HIV-2 RT at concentrations as high as 500 μg/ml (Table 4).
TABLE 4.
Inhibitory effects of pyridine oxide derivatives on HIV-1 RT and HIV-2 RT activity in the presence of different homopolymeric template-primers
| Compound | IC50 (μg/ml)a
|
|||
|---|---|---|---|---|
| HIV-1 RT
|
HIV-2 RT with poly(rC · dG) | |||
| Poly(rC · dG) | Poly(rA · dT) | Poly(rI · dC) | ||
| JPL-10 | 107 ± 10 | 130 ± 7 | >500 | >500 |
| JPL-27 | 132 ± 25 | 161 ± 19 | >500 | >500 |
| JPL-30 | 94 ± 12 | 123 ± 34 | >500 | >500 |
| JPL-71 | 5.2 ± 2.1 | 4.7 ± 1.7 | 227 ± 109 | >500 |
| JPL-74 | 14 ± 1 | 8.6 ± 3.1 | >500 | >500 |
| JPL-133 | 2.4 ± 0.7 | 1.8 ± 0.6 | 92 ± 8 | >500 |
IC50, compound concentration required to inhibit the incorporation of radiolabeled dNTP into homopolymeric templates by 50%. Data represent the average values (± standard deviation) for at least two independent experiments.
FIG. 1.
Log IC50 for inhibition of HIV-1 RT activity [using poly(rC · dG) as the template and dGTP as the radiolabeled substrate] versus log EC50 for inhibition of HIV-1(IIIB) replication in CEM cell cultures.
Genotypic analysis of the RT of mutant HIV-1 strains selected in the presence of pyridine oxide derivatives.
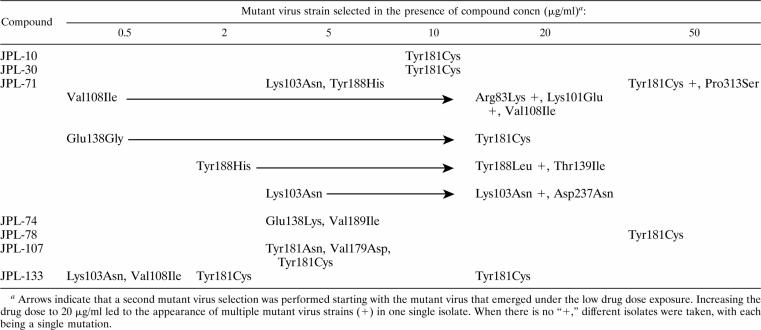
HIV-1(IIIB)-infected CEM cell cultures were exposed to a variety of pyridine oxide derivatives at different fixed concentrations (Table 5). After a few passages, breakthrough of viral cytopathicity could be recorded in most cases. The majority of pyridine oxide derivatives resulted in the appearance of the HIV-1 Tyr181Cys RT mutation, especially at the higher drug concentrations. At lower initial drug concentrations, other amino acid mutations were observed, including Lys103Asn, Val108Ile, and Tyr188His. Increasing the JPL-71 concentration after virus emergence in the presence of the initial drug concentration led to the appearance of the Lys101Glu RT mutation in combination with the Val108Ile RT mutation. We also observed the appearance of a novel Asp237Asn RT mutation in combination with the Lys103Asn RT mutation, whereas the Tyr188His RT mutant virus shifted to a Tyr188Leu RT mutant and additionally acquired the Thr139Ile RT mutation. It should be noted that the additional mutations appeared only after a marked number of subcultivations. A Glu138Lys RT mutation emerged mostly after treatment of HIV-1(IIIB)-infected CEM cell cultures with JPL-74 regardless of the drug concentration used.
TABLE 5.
Genotypic analysis of the RT of mutant HIV-1 strains after exposure to pyridine oxide derivatives
Analysis of the RT gene of 22 independent resistant HIV-1(IIIB) strains revealed that the codon mutations that appeared in the RT gene were mostly due to transition of guanine to adenine (G→A) or transition of adenine to guanine (A→G) (Table 6). Several RT mutants were also formed through transversion of adenine to thymine (A→T) or transition of cytosine to thymine (C→T) or vice versa (T→A or T→C). No mutations emerged through conversion of guanine to thymine (G→T), cytosine to adenine (C→A), cytosine to guanine (C→G), or vice versa (T→G, A→C, or G→C).
TABLE 6.
Genotypic analysis of the RT mutations that appeared in 22 independently obtained HIV-1(IIIB) mutants in CEM cell cultures
| Mutation | Silent mutation(s)
|
Non-NNRTI-characteristic mutation(s)
|
NNRTI-characteristic mutation(s)
|
|||
|---|---|---|---|---|---|---|
| No.a | Type | No.a | Type | Total no.a | Type (no.) | |
| A → T | 1 | Ala98Ala | 0 | 4 | Lys103Asn (3), Tyr188Leu (1) | |
| C → T | 4 | Phe171Phe | 0 | 2 | Thr139Ile (1), Pro313Ser (1) | |
| T → A | 0 | 0 | 2 | Val179Asp (1), Tyr181Asn (1) | ||
| T → C | 2 | Asn136Asn | 6 | Ile165Thr | 2 | Tyr188His (1), Tyr188Leu (1) |
| G → A | 6 | Glu53Glu, Val111Val | 0 | 9 | Glu138Lys (3), Val108Ile (3), Val189Ile (1), Asp237Asn (1), Arg83Lys (1) | |
| A → G | 1 | Lys275Lys | 0 | 10 | Tyr181Cys (8), Glu138Gly (1), Lys101Glu (1) | |
Numbers represent the total number of silent or non-NNRTI-characteristic mutations that emerged through the transition or transversion indicated at the left. No mutations emerged through conversion of guanine to thymine (G → T), cytosine to adenine (C → A), or cytosine to guanine (C → G) or vice versa (T → G, A → C, or G → C).
Inhibitory effect of JPL derivatives against mutant HIV-1 strains in CEM cell cultures.
We evaluated several mutant HIV-1 strains that emerged in the presence of the pyridine oxide derivatives and other mutant HIV-1 strains, containing NNRTI-characteristic mutations in the RT, for their susceptibilities to the different pyridine oxide derivatives (Table 7). The NNRTI nevirapine, included as control agent, had a markedly reduced inhibitory potency against several of the mutant virus strains, up to 300-fold depending on the nature of the amino acid substitution in the RT of the mutant virus strains. The NNRTIs efavirenz and the thiocarboxanilide UC-781 also lost inhibitory potency against these mutant viruses but to a lesser extent (30-fold at most). Pyridine oxide derivatives markedly lost their inhibitory activities against the HIV-1 strains that emerged under pyridine oxide drug treatment. The Tyr181Cys mutation, which appeared most frequently in the presence of the higher pyridine oxide concentrations in the HIV-1(IIIB)-infected CEM cell cultures, obviously resulted in a marked decrease of the antiviral potency of these compounds. Whereas the most potent NNRTI pyridine oxide, JPL-133, was not able to inhibit HIV-1-induced cytopathicity at 20 μg/ml when the Tyr181Cys mutation was present in the mutant RT, it retained marked antiviral potency against other NNRTI-characteristic RT mutants, such as Leu100Ile and Val179Asp RT mutant virus. Interestingly, JPL-10, JPL-27, and JPL-30 showed only a relatively minor decrease in the antiviral activity against all mutant HIV-1 strains that contained NNRTI-specific mutations. As expected, NRTIs and the nucleoside phosphonate RT inhibitor tenofovir retained full antiviral activity against all mutant HIV-1 strains, irrespective of the nature of the amino acid mutation in the HIV-1 RT (Table 7).
TABLE 7.
Sensitivity of mutant HIV-1(IIIB) strains towards various HIV-1 RT inhibitors
| Compound | EC50 (μg/ml)a
|
||||||
|---|---|---|---|---|---|---|---|
| Wild type | Tyr181Cys (JPL-71)c | Lys103Asn (JPL-71)c | Glu138Lys (JPL-74)c | Tyr188His (JPL-71)c | Val179Asp (JPL-107)c | Leu100Ile | |
| Nevirapine | 0.006 ± 0.001 | 2.2 ± 0.9 | 1.8 ± 0.5 | 0.04 ± 0.02 | 0.02 ± 0.002 | 0.02 ± 0.001 | 0.06 ± 0.02 |
| Efavirenz | 0.0006 ± 0.0001 | 0.003 ± 0.001 | 0.02 ± 0.001 | 0.003 ± 0.001 | 0.002 ± 0.001 | 0.002 ± 0.001 | 0.01 ± 0.007 |
| UC-781 | 0.002 ± 0.001 | 0.06 ± 0.02 | 0.07 ± 0.04 | 0.007 ± 0.004 | 0.012 ± 0.001 | 0.003 ± 0.001 | 0.02 ± 0.008 |
| AZT | 0.002 ± 0.0008 | 0.001 ± 0.0003 | 0.005 ± 0.001 | 0.003 ± 0.0008 | 0.001 ± 0.0003 | 0.001 ± 0.0003 | 0.002 ± 0.0005 |
| 3TC | 0.03 ± 0.02 | 0.02 ± 0.001 | 0.03 ± 0.008 | 0.02 ± 0.004 | 0.01 ± 0.004 | 0.02 ± 0.003 | 0.03 ± 0.006 |
| Tenofovir | 1.0 ± 0.8 | 1.1 ± 0.4 | 1.7 ± 0.2 | 1.2 ± 0.5 | 0.8 ± 0.6 | 0.9 ± 0.6 | 1.1 ± 0.4 |
| JPL-10 | 5.3 ± 2.6 | 20 ± 6 | 11 ± 5 | 12 ± 4 | 8.8 ± 1.1 | 8.0 ± 0.1 | 10 ± 2 |
| JPL-27 | 6.8 ± 3.5 | >20 | 16 ± 6 | ≥20 | ≥20 | ||
| JPL-30 | 5.9 ± 3.0 | 50 ± 4 | 44 ± 4 | 48 ± 24 | 40 ± 8 | ||
| JPL-71 | 0.10 ± 0.01 | ≥20 | 12 ± 1 | 4 ± 3 | 8.8 ± 1.1 | 0.35 ± 0.09 | 4.6 ± 2.1 |
| JPL-74 | 0.48 ± 0.01 | >4b | >4b | >4b | >4b | ||
| JPL-78 | 3.4 ± 0.9 | 50 ± 23 | 50 ± 25 | 60 ± 22 | 60 ± 26 | ||
| JPL-107 | 0.52 ± 0.03 | >4b | >4b | >4b | 2.4 ± 0.4 | >4b | >4b |
| JPL-110 | 9.6 ± 1.4 | 60 ± 25 | 48 ± 24 | 60 ± 4 | 50 ± 21 | ||
| JPL-133 | 0.05 ± 0.01 | ≥20 | 4.8 ± 1.9 | 1.2 ± 0.8 | 9.2 ± 1.7 | 0.32 ± 0.04 | 0.2 ± 0.1 |
| JPL-146 | 2.2 ± 0.2 | ≥20 | 9.6 ± 1.1 | ≥20 | 1.9 ± 0.7 | ||
| JPL-150 | 4.0 ± 0.1 | >20 | >20 | >20 | 10 ± 1 | ||
EC50, compound concentration required to inhibit HIV-1-induced cytopathicity (giant cell formation) in CEM cell cultures. Data represent the average values (± SD) for at least three independent experiments.
Higher drug concentrations could not be accurately determined due to insolubility of the compound at these concentrations.
HIV-1 strain that emerged upon exposure to the pyridine oxide derivative indicated in parentheses.
DISCUSSION
The pyridine oxide derivatives represent an entirely new class of anti-HIV compounds. It is quite remarkable that some of the pyridine oxide derivatives specifically inhibit HIV-1 replication, whereas other compounds are able to inhibit both HIV-1 and HIV-2 strains. Also, we have recently showed that a variety of pyridine oxide derivatives were also able to suppress cytomegalovirus (CMV) infection in cell culture (5). This is an unusual anti-HIV/CMV spectrum that has not been reported for any other NNRTI-like compound. The most active pyridine oxide congener yet discovered (JPL-133) proved to be inhibitory to HIV-1(IIIB) replication in CEM cell cultures at an EC50 of 0.05 μg/ml without being toxic for the CEM cells at a 50% cytostatic concentration of 38 μg/ml, thus resulting in a selectivity index of ∼760. Similar EC50 values were found when JPL-133 was evaluated in other cell lines or against HIV-1 strains other than IIIB.
All pyridine oxide derivatives, both those that were HIV-1-specific and those that were inhibitory against HIV-1 and HIV-2, selected for NNRTI-characteristic mutations within the first six subcultivations. This speed of emergence of mutant HIV-1 strains is comparable to the speed reported earlier for NNRTIs such as nevirapine, TIBO, pyridinone, delavirdine, or TSAO derivatives to elicit virus-mediated drug resistance (2, 3). At higher drug concentrations, the Tyr181Cys RT mutant was predominantly selected, whereas lower drug concentrations rather resulted in the appearance of a larger variety of NNRTI-characteristic mutations, such as Lys103Asn, Val108Ile, and Tyr188His. Also, exposure to increasing drug concentrations resulted in the appearance of additional RT mutations (i.e., Lys103Asn/Asp237Asn and Tyr188Leu/Thr139Ile double-mutant viruses and even an Arg83Lys/Val108Ile/Lys101Glu triple mutant), resulting in full resistance to the particular pyridine oxides. Several unusual mutations were observed: Tyr181Asn has never been reported before, and Pro313Ser, Asp237Asn, and Arg83Lys have never been previously associated with NNRTI resistance. It is currently unclear what, if any, role these amino acid mutations play in viral resistance to the pyridine oxide derivatives, since they invariably appeared in the presence of other NNRTI-specific mutations.
Genotypic analysis of the mutant HIV-1 RTs of 22 independent drug-resistant HIV-1 strains revealed the predominant appearance of the transition mutations G→A and A→G (Table 6). G→A (hyper)mutations in the HIV-1 RT gene have been directly linked to a dCTP pool imbalance during reverse transcription (10, 13, 14) and have been shown to occur in the codons for Arg83Lys (AGA→AAA), Val108Ile (GTA→ATA), Glu138Lys (GAG→AAG), Val189Ile (GTA→ATA), and Asp237Asn (GAT→AAT). In contrast, the A→G transition mutation most prominently occurred in the codon for Tyr181Cys (TAT→TGT). Among the silent mutations that emerged under drug pressure, the G→A transition was again predominant, followed by the C→T transition, which, in turn, appeared more frequently than the A→G transition mutation.
Remarkably, JPL-10 and JPL-30, which are inhibitory to both HIV-1 and HIV-2 replication, also selected for NNRTI-specific mutations in the HIV-1 RT. Whereas there was a close correlation between inhibition of HIV-1 RT activity and antiviral activity of all pyridine oxide derivatives evaluated (r = 0.98) (Fig. 1), none of the pyridine oxide derivatives, including JPL-10, JPL-27, and JPL-30, inhibited HIV-2 RT. Poly(rC ·dG) and dGTP have been routinely used as the most optimal homopolymeric template-primer and substrate for HIV-1 RT inhibition measurements in the presence of NNRTIs. It cannot be excluded that due to the artificial template-primer used to measure RT inhibition, the RT inhibitory values are not fully reflecting the RT inhibition in the intact virus-infected cells. However, it should be noted that a very close correlation was found between anti-HIV-1 inhibition and anti-HIV-1 RT inhibition (r = 0.98), pointing to the likely relevance of our findings. Clearly, the latter compounds exert their inhibitory activity against HIV-1 by interfering with RT, but their activity against HIV-2 may be attributed to another mechanism. Also, the fact that these pyridine oxide derivatives partially retained anti-HIV-1 activity against the virus strains that harbored NNRTI-specific mutations suggests that this residual anti-HIV-1 activity may be ascribed to another mode of antiviral action common for HIV-1 and HIV-2. One possibility to explain the additional anti-HIV-2 activity of a number of pyridine oxide derivatives is that this activity is due to toxicity of the test compounds. However, in contrast with many toxic pyridine oxide derivatives where abundant giant cell formation was observed at partially toxic compound concentrations (i.e., at their 50% cytotoxic concentration [CC50] values), marked suppression of virus-induced giant cell formation was noted for several other pyridine oxide derivatives at their CC50 values (i.e., JPL-10, JPL-27, and JPL-30). Moreover, the anti-CMV activity of JPL-10, JPL-27, and JPL-30 (5) in addition to their anti-HIV-1 and HIV-2 activity may rather lead to the hypothesis that pyridine oxide derivatives—at least those that show activity against both HIV-1 and HIV-2—may be targeted at a cellular event that may be in common between HIV and CMV and may be required for efficient HIV and CMV replication in cell culture.
It should be mentioned that liver microsomes may easily oxidize sulfide derivatives to sulfoxides and subsequently to sulfones. Several antivirally active pyridine oxides are in the sulfide form (i.e., JPL-133), but other active compounds are in the sulfone form (i.e., JPL-71). An extensive structure-activity relation study for almost 200 pyridine oxides revealed that oxidation of the sulfides to sulfoxides or sulfones does not necessarily result in decreased antiviral activity (5). Therefore, it is expected that this particular liver metabolic activity will not compromise the antiviral potential of most of these drugs.
In conclusion, we have described an entirely new class of HIV inhibitors for which several members of the class of pyridine oxide derivatives display specific anti-HIV-1 activity, whereas others are endowed with both anti-HIV-1 and -HIV-2 activity. These compounds have been accredited with a dual mechanism of antiviral action. These findings, together with the huge number of possible modifications that can be introduced in the chemical structure of the flexible pyridine oxide derivatives, open interesting perspectives to develop more potent and selective anti-HIV congeners and to further exploit and unravel their dual mechanism of antiviral action.
Acknowledgments
We are indebted John Lacadie and James Pierce of Crompton Corporation (Middlebury, Conn.) for supplying the compounds used in this study. We are grateful to Ann Absillis and Lizette van Berckelaer for excellent technical assistance and to Christiane Callebaut for fine editorial help.
Financial support by the Belgian Fonds voor Wetenschappelijk Onderzoek (FWO project no. G.0104.98), the Belgian Geconcerteerde Onderzoeksacties (project no. GOA-00/12), Crompton Corporation (Middlebury, Conn.), and the European commission (Krediet no. QLRT 2000-00291 and 2001-01311 and the Rene Descartes Prize 2001 no. HPAW-2002-90001) is gratefully acknowledged.
REFERENCES
- 1.Balzarini, J., M.-J. Pérez-Pérez, A. San-Félix, M.-J. Camarasa, I. C. Bathurst, P. J. Barr, and E. De Clercq. 1992. Kinetics of inhibition of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase by a novel HIV-1-specific nucleoside analogue [2′, 5′-bis-O-(tert-butyldimethylsilyl)-β-D-ribofuranosyl]-3′-spiro-5′′-(4′′-amino-1′′, 2′′-oxathiole-2′′, 2′′-dioxide)thymine (TSAO-T). J. Biol. Chem. 267:11831-11838. [PubMed] [Google Scholar]
- 2.Balzarini, J., A. Karlsson, M.-J. Pérez-Pérez, M.-J. Camarasa, W. G. Tarpley, and E. De Clercq. 1993. Treatment of human immunodeficiency virus type 1 (HIV-1)-infected cells with combinations of HIV-1-specific inhibitors result in a different resistance pattern than does treatment with single-drug therapy. J. Virol. 67:5353-5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balzarini, J., A. Karlsson, M.-J. Pérez-Pérez, L. Vrang, J. Walbers, H. Zhang, B. Öberg, A.-M. Vandamme, M.-J. Camarasa, and E. De Clercq. 1993. HIV-1-specific reverse transcriptase inhibitors show differential activity against HIV-1 mutant strains containing different amino acid substitutions in the reverse transcriptase. Virology 192:246-253. [DOI] [PubMed] [Google Scholar]
- 4.Balzarini, J. 1999. Suppression of resistance to drugs targeted to human immunodeficiency virus reverse transcriptase by combination therapy. Biochem. Pharmacol. 58:1-27. [DOI] [PubMed] [Google Scholar]
- 5.Balzarini, J., M. Stevens, G. Andrei, R. Snoeck, R. Strunk, J. B. Pierce, J. A. Lacadie, E. De Clercq, and C. Pannecouque. 2002. Pyridine oxide derivatives: structure-activity relationship for inhibition of human immunodeficiency virus and cytomegalovirus replication in cell culture. Helvetica Chimica Acta 85:2961-2974. [Google Scholar]
- 6.Barré-Sinoussi, F., J. C. Chermann, F. Rey, M. T. Nugeyre, S. Chamaret, J. Gruest, C. Dauguet, C. Axler-Blin, F. Vézinet-Brun, C. Rouzioux, W. Rozenbaum, and L. Montagnier. 1983. Isolation of a T-lymphotropic retrovirus from a patient at risk for AIDS. Science 220:868-871. [DOI] [PubMed] [Google Scholar]
- 7.De Clercq, E. 1999. Perspectives of non-nucleoside reverse transcriptase inhibitors (NNRTIs) in the therapy of HIV-1 infection. Il Farmaco. 54:26-45. [DOI] [PubMed] [Google Scholar]
- 8.De Clercq, E. 2002. New anti-HIV agents and targets. Med. Res. Rev. 22:531-565. [DOI] [PubMed] [Google Scholar]
- 9.Esnouf, R., J. Ren, C. Ross, Y. Jones, D. Stammers, and D. Stuart. 1995. Mechanism of inhibition of HIV-1 reverse transcriptase by non-nucleoside inhibitors. Nat. Struct. Biol. 2:303-308. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgibbon, J. E., S. Mazar, and D. T. Dubin. 1993. A new type of G→A hypermutation affecting human immunodeficiency virus. AIDS Res. Hum. Retrovir. 9:833-838. [DOI] [PubMed] [Google Scholar]
- 11.Popovic, M., M. G. Sarngadharan, E. Read, and R. C. Gallo. 1984. Detection, isolation and continuous production of cytopathic retrovirus (HTLV-III) from patients with AIDS and pre-AIDS. Science 224:497-500. [DOI] [PubMed] [Google Scholar]
- 12.Rey, M.-A., B. Kurst, A. G. Laurent, D. Guétard, L. Montagnier, and A. G. Hovanessian. 1989. Characterization of an HIV-2-related virus with a smaller sized extra-cellular envelope glycoprotein. Virology 173:258-267. [DOI] [PubMed] [Google Scholar]
- 13.Vartanian J.-P., A. Meyerhans, B. Åajö, and S. Wain-Hobson. 1991. Selection, recombination, and G→A hypermutation of human immunodeficiency virus type 1 genomes. J. Virol. 65:1779-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vartanian J.-P., A. Meyerhans, M. Sala, and S. Wain-Hobson. 1994. G→A hypermutation of the human immunodeficiency virus type 1 genome: evidence for dCTP pool imbalance during reverse transcription. Proc. Natl. Acad. Sci. USA 91:3092-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]