Abstract
A new antibiotic resistance gene cluster comprising genes for sulfonamide (sul2), streptomycin (strA-strB), and tetracycline [tetR-tet(H)] resistance was detected on plasmid pVM111 from Pasteurella multocida. The tetR-tet(H) gene region was inserted between sul2 and strA, possibly by illegitimate recombination. Two potential recombination sites of 18 and 25 bp were identified.
The first tetracycline resistance (tet) gene of hybridization class H was detected in 1993 on plasmid pVM111 (4) from a Pasteurella multocida isolate obtained in 1975 from the tissues of a turkey in California that had died of avian cholera (5). Later, tet(H) genes were also detected in porcine and bovine P. multocida and Mannheimia haemolytica isolates (3). In 1998, the tet(H) gene was identified as part of the composite transposon Tn5706 from P. multocida (12). In recent years, three types of tet(H)-carrying plasmids, designated pPMT1 (12), pPAT1 (8), and pMHT1 (7), have been analyzed in detail. All these plasmids were detected in either P. multocida, Pasteurella aerogenes, or various Mannheimia sp. isolates from cattle or pigs. They were 4.4 to 6.8 kb in size and mediated only tetracycline resistance. While restriction maps and sequence data for the regions flanking the tetR-tet(H) gene region were available for these plasmids, the corresponding data are still missing for pVM111. Since plasmid pVM111 has been found to be larger than the other tet(H)-carrying plasmids known so far and has also been found to mediate sulfonamide and streptomycin resistance by genes that have not been further specified (4), we analyzed plasmid pVM111 for its resistance genes and their organization with regard to cotransfer of the resistance genes and its impact on the development and spread of multiresistance.
Plasmid pVM111 was transformed into Escherichia coli JM109 by the CaCl2 method, and transformants were selected on Luria-Bertani agar supplemented with 20 μg of tetracycline/ml. Pure plasmid DNA suitable for restriction mapping, PCR analysis, cloning experiments, and sequence analysis was obtained by alkaline lysis with subsequent purification by affinity chromatography on Midi columns (Qiagen, Hilden, Germany) (9). Restriction mapping already suggested that pVM111 does not carry a complete copy of Tn5706. Confirmation of the presence of the tet(H) gene was done by PCR (3). PCR-directed analysis of the genes responsible for sulfonamide and streptomycin resistance (10, 11) revealed the presence of the genes sul2 and strA in pVM111.
In previous studies with isolates of P. aerogenes, M. haemolytica, Mannheimia varigena, and Mannheimia taxon 10 from cattle and swine, the primers suitable for the detection of a linkage of the genes sul2 and strA led to the identification of a first resistance gene cluster in which a catA3 gene for chloramphenicol resistance was inserted into the noncoding spacer between sul2 and strA (10, 11). The same approach with the primer combination sul1 and str2 (the sequence of the forward primer was from the 5′ end of sul2, and the sequence of the reverse primer was from the 3′ end of strA) (10) and pVM111 DNA as the template resulted in an amplicon of ca. 3.5 kb. When this amplicon was used as a template for PCR analysis, positive results were seen not only for sul2 and strA but also for tet(H), suggesting that a tet(H) segment might have been inserted between sul2 and strA.
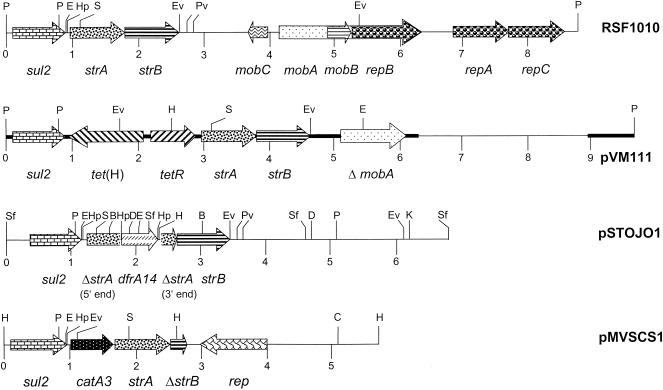
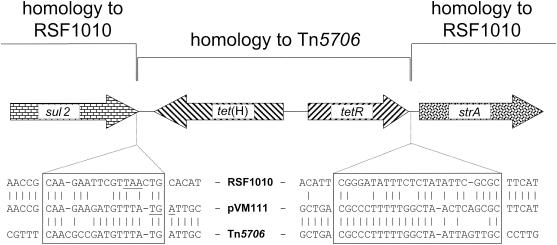
For sequence analysis, the two ca. 4.5-kb EcoRI-PstI fragments and the 0.8-kb PstI fragment (Fig. 1) were cloned into pBluescript II SK(+) (Stratagene, Amsterdam, The Netherlands). Both strands of the small PstI fragment and the EcoRI-PstI fragment which contained the tetR-tet(H) region as well as the strA-strB genes were sequenced completely, while fragments of approximately 850 bp at both ends of the second EcoRI-PstI fragment were sequenced (Fig. 1). In total, the sequence of a ca. 7-kb segment of the 9.8-kb plasmid pVM111 was determined by primer walking starting with M13 universal and reverse primers (Stratagene). Analysis of the sequence showed the arrangement of the resistance genes displayed in Fig. 1. Studies of the sequence at the junctions between the tetR-tet(H) gene region and the adjacent sul2 and strA upstream sequences suggested that integration of the tet gene region might have occurred by illegitimate recombination. Two possible recombination sites of 18 and 25 bp were identified (Fig. 2). Recombination between the noncoding region downstream of tet(H) in Tn5706 with the terminal sul2 sequence resulted in an extension of the sul2 reading frame by one codon, in addition to the change of the final three codons of sul2 in pVM111 (Fig. 2). Variations in the terminal parts of the sul2 reading frame which caused either an extension or a shortening of the sul2 reading frame but which did not affect the biological activity of the corresponding dihydropteroate synthase have previously been observed in plasmids pLS88 from Haemophilus ducreyi (2) and pYFC1 from “Pasteurella haemolytica” (1). The sequence of the 18-bp recombination site (Fig. 2) showed 67% identity to the sequence of sul2-strA-strB-carrying plasmid RSF1010 (14) and 78% identity to the corresponding part of the Tn5706 sequence (12). The second recombination site of 25 bp was located in the noncoding spacer downstream of tetR and upstream of strA. It showed 64% identity to the RSF1010 sequence and 76% identity to the noncoding region downstream of tetR in Tn5706. The sequence of the tetR-tet(H) gene region of pVM111 corresponded exactly to that previously described by Hansen et al. (4). The sequences of the flanking parts corresponded closely to the sequence of broad-host-range plasmid RSF1010 (14).
FIG. 1.
Comparative analysis of restriction maps and structural organizations of plasmids RSF1010 (14), pVM111 (this study), pSTOJO1 (13), and pMVSCS1 (11). Restriction endonucleases are abbreviated as follows: B, BclI; D, DraI; E, EcoRI; Ev, EcoRV; H, HindIII; Hp, HpaI; K, KpnI; P, PstI; Pv, PvuII; S, SacI; and Sf, SfuI. A distance scale (in kilobases) is presented below each map. The reading frames for genes sul2, strA, ΔstrA, strB, ΔstrB, tet(H), tetR, dfrA14, catA3, mobA to mobC, ΔmobA, rep, and repA to repC are shown as arrows, with the direction of transcription indicated by the arrowhead. The black bar in the map of pVM111 indicates the sequenced part.
FIG. 2.
Presentation of the two potential recombination sites downstream of tet(H) and tetR probably involved in the integration of a Tn5706-like tetR-tet(H) gene region in an RSF1010-like spacer region between sul2 and strA. Identical bases with respect to the pVM111 sequence are indicated by vertical bars. The original translational stop codon of the sul2 gene in the RSF1010 sequence and the alternative stop codon in the pVM111 sequence are underlined. The two putative recombination sites are displayed as boxes.
Multiresistance gene clusters are of particular importance since they confer resistance to several different antimicrobials or classes of antimicrobials, e.g., sulfonamides, chloramphenicol, and streptomycin (10, 11) or sulfonamides, tetracyclines, and streptomycin. When such resistance gene clusters are located on plasmids, they are easily spread among strains, species, and sometimes even genera. The spread of a plasmid carrying a multiresistance gene cluster bears the danger of the coselection and persistence of resistance genes even without direct selective pressure. Previous studies have shown that sul2-strA-carrying plasmids are widespread among gram-negative bacteria (10, 16, 17) and that they are capable of accepting other resistance genes, such as catA3 (10, 11) and dfrA14 (13), to form new resistance gene clusters (Fig. 1). These observations were extended in this study by the description of a new type of plasmid-borne antibiotic resistance gene cluster in P. multocida. Resistance to sulfonamides, streptomycin, or tetracyclines had been reported to occur at high frequencies of 72.6, 50.0, and 40.5%, respectively, among P. multocida isolates from Germany (6); and resistance to these antimicrobials represented the most prevalent type of resistance in these bacteria. PCR screening of a large number of independent Pasteurella and Mannheimia isolates from food-producing animals confirmed that this new type of resistance gene cluster has not yet been observed in European Pasteurella and Mannheimia isolates (10, 11). However, the finding that approximately one-third of the sul2-strA-carrying isolates identified in recent studies (10, 11) also harbored tet(H) genes (7-9) might indicate the potential for the development of such a cluster in European isolates as well. For this, the widespread use of tetracyclines, which account for almost two-thirds of all antimicrobials used in veterinary medicine in the European Union member states and Switzerland (15), might represent a relevant selective force. In addition, the observation that plasmids from Pasteurella and Mannheimia carrying any type of sul2-strA-based resistance gene cluster known so far can replicate and express their resistance genes not only in their original hosts but also in E. coli underlines the importance of these plasmids in the spread of antimicrobial multiresistance.
Nucleotide sequence accession number.
The sequence of the 7,006-bp segment of pVM111 has been deposited in the EMBL database under accession number AJ514834.
Acknowledgments
We thank Jean-Marc Collard for providing plasmid pVM111.
Nga Thi Thu Tham was financially supported by the Carl-Duisberg-Society.
REFERENCES
- 1.Chang, Y. F., D. P. Ma, H. Q. Bai, R. Young, D. K. Struck, S. J. Shin, and D. H. Lein. 1992. Characterization of plasmids with antimicrobial resistant genes in Pasteurella haemolytica. DNA Sequence 3:89-97. [DOI] [PubMed] [Google Scholar]
- 2.Dixon, L. G., W. L. Albritton, and P. J. Willson. 1994. An analysis of the complete nucleotide sequence of the Haemophilus ducreyi broad-host-range plasmid pLS88. Plasmid 32:228-232. [DOI] [PubMed] [Google Scholar]
- 3.Hansen, L. M., P. C. Blanchard, and D. C. Hirsh. 1996. Distribution of tet(H) among Pasteurella isolates from the United States and Canada. Antimicrob. Agents Chemother. 40:1558-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansen, L. M., L. M. McMurray, S. B. Levy, and D. C. Hirsh. 1993. A new tetracycline resistance determinant, Tet H, from Pasteurella multocida specifying active efflux of tetracycline. Antimicrob. Agents Chemother. 37:2699-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirsh, D. C., L. D. Martin, and K. R. Rhoades. 1985. Resistance plasmids of Pasteurella multocida isolated from turkeys. Am. J. Vet. Res. 46:1490-1493. [PubMed] [Google Scholar]
- 6.Hörmansdorfer, S., and J. Bauer. 1996. Zur Resistenzsituation boviner Pasteurellen (Resistance pattern of bovine pasteurellae). Berl. Münch. Tierärztl. Wochenschr. 109:168-171. (In German.) [PubMed]
- 7.Kehrenberg, C., S. A. Salmon, J. L. Watts, and S. Schwarz. 2001. Tetracycline resistance genes in isolates of Pasteurella multocida, Mannheimia haemolytica, Mannheimia glucosida, and Mannheimia varigena from bovine and swine respiratory disease: intergeneric spread of plasmid pMHT1. J. Antimicrob. Chemother. 48:631-640. [DOI] [PubMed] [Google Scholar]
- 8.Kehrenberg, C., and S. Schwarz. 2000. Identification of a truncated, but functionally active tet(H) tetracycline resistance gene in Pasteurella aerogenes and Pasteurella multocida. FEMS Microbiol. Lett. 188:191-195. [DOI] [PubMed] [Google Scholar]
- 9.Kehrenberg, C., and S. Schwarz. 2001. Molecular analysis of tetracycline resistance in Pasteurella aerogenes. Antimicrob. Agents Chemother. 45:2885-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kehrenberg, C., and S. Schwarz. 2001. Occurrence and linkage of genes coding for resistance to sulfonamides, streptomycin and chloramphenicol in bacteria of the genera Pasteurella and Mannheimia. FEMS Microbiol. Lett. 205:283-290. [DOI] [PubMed] [Google Scholar]
- 11.Kehrenberg, C., and S. Schwarz. 2002. Nucleotide sequence and organization of plasmid pMVSCS1 from Mannheimia varigena: identification of a multiresistance gene cluster. J. Antimicrob. Chemother. 49:383-386. [DOI] [PubMed] [Google Scholar]
- 12.Kehrenberg, C., C. Werckenthin, and S. Schwarz. 1998. Tn5706, a transposon-like element from Pasteurella multocida mediating tetracycline resistance. Antimicrob. Agents Chemother. 42:2116-2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ojo, K. K., C. Kehrenberg, S. Schwarz, and H. A. Odelola. 2002. Identification of a complete dfrA14 gene cassette integrated at a secondary site in a resistance plasmid of uropathogenic Escherichia coli from Nigeria. Antimicrob. Agents Chemother. 46:2054-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scholz, P., V. Haring, B. Wittmann-Liebold, K. Ashman, M. Bagdasarian, and E. Scherzinger. 1989. Complete nucleotide sequence and gene organization of the broad-host-range plasmid RSF1010. Gene 75:271-288. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz, S., and E. Chaslus-Dancla. 2001. Use of antimicrobials in veterinary medicine and mechanisms of resistance. Vet. Res. 32:201-225. [DOI] [PubMed] [Google Scholar]
- 16.Sundin, G. W. 2000. Examination of base pair variants of the strA-strB streptomycin resistance genes from bacterial pathogens of humans, animals and plants. J. Antimicrob. Chemother. 46:848-849. [DOI] [PubMed] [Google Scholar]
- 17.Sundin, G. W., and C. L. Bender. 1996. Dissemination of the strA-strB streptomycin resistance genes among commensal and pathogenic bacteria from humans, animals and plants. Mol. Ecol. 5:133-143. [DOI] [PubMed] [Google Scholar]