Abstract
We employed a murine model to test the concept of using an aerosolized, long-acting antiviral drug to protect humans against smallpox. We previously showed that a low dose of aerosolized cidofovir (HPMPC [Vistide]) was highly protective against subsequent aerosolized cowpox virus challenge and was more effective than a much larger dose of drug given by injection, suggesting that aerosolized cidofovir is retained in the lung. Because the nephrotoxicity of cidofovir is a major concern in therapy, delivering the drug directly to the respiratory tract might be an effective prophylactic strategy that maximizes the tissue concentration at the site of initial viral replication, while minimizing its accumulation in the kidneys. In the present study, we found that treating mice with aerosolized 14C-labeled cidofovir (14C-cidofovir) resulted in the prolonged retention of radiolabeled drug in the lungs at levels greatly exceeding those in the kidneys. In contrast, subcutaneous injection produced much higher concentrations of 14C-cidofovir in the kidneys than in the lungs over the 96-h time course of the study. As further evidence of the protective efficacy of aerosolized cidofovir, we found that aerosol treatment before or after infection was highly protective in mice challenged intranasally with cowpox virus. All or nearly all mice that were treated once by aerosol, from 2 days before to 2 days after challenge, survived intranasal infection, whereas all placebo-treated animals died.
Protection against smallpox has traditionally been based on vaccination with vaccinia virus, a method that led to the global eradication of the disease in the late 1970s. The subsequent discontinuation of vaccination has rendered most of the world's population susceptible to smallpox. The current public health response plan to a smallpox bioterrorist attack calls for the rapid organization of mass vaccination programs. It is not certain that such measures could always be effectively implemented under the disruptive conditions of such an event. In addition, vaccination poses a significant risk to large numbers of individuals with immunodeficiency disorders or certain common skin conditions (1). For these reasons, it would clearly be desirable to supplement the traditional method of prophylaxis with a long-acting antiviral medication that could be self-administered under outbreak conditions and would provide immediate resistance to smallpox infection.
We have been exploring such an approach, based on aerosol delivery of the antiviral drug cidofovir {(S)-1-[3-hydroxy-2-(phosphonylmethoxy)propyl]cytosine [HPMPC; Vistide]} (2). Cidofovir is licensed for clinical use for the treatment of cytomegalovirus retinitis in AIDS patients and is active against a range of DNA viruses, including herpesvirus, adenovirus, polyomavirus, and papillomavirus (23). The compound is a nucleoside phosphonate, an analog of dCMP, that acts by inhibiting the viral DNA polymerase (8, 9, 18). Its 3-day intracellular half-life means that a single dose can provide a lengthy period of protection (14). In addition to the above-noted range of antiviral activity, cidofovir potently inhibits a range of orthopoxviruses, including the agent of smallpox, variola virus (1, 8, 9, 20, 21).
We earlier employed a weanling mouse model of aerosolized cowpox virus infection to demonstrate that a single subcutaneous (s.c.) injection of cidofovir given up to a week before or several days after viral challenge protected mice against death (3, 17). However, in the case of smallpox infection, the delivery of cidofovir directly to the respiratory tract either before or soon after infection would seem to be a rational approach when one considers that the disease is transmitted both by primary aerosols (12, 13) and by particles expelled from infected patients (10, 25). We therefore went on to evaluate the efficacy of aerosolized cidofovir in the cowpox model and found that a low dose prevented the development of all signs of infection after aerosolized virus challenge, suggesting that aerosolized cidofovir was taken up and retained in cells of the respiratory tract (2).
In the present study, we examined the question of the tissue distribution of aerosolized cidofovir by delivering radiolabeled drug to mice by either the aerosol or subcutaneous route. Because cidofovir treatment of humans has occasionally been associated with nephrotoxicity, our analysis focused on comparison of the relative concentrations of radiolabeled drug in the lungs and kidneys over time after administration by the two different routes. We found that aerosol delivery resulted in the prolonged retention of cidofovir in the lungs, at much higher concentrations than in the kidneys, whereas subcutaneous injection produced the opposite effect. We also tested the ability of the aerosolized drug to protect mice against upper airway infection by challenging them intranasally with a lethal dose of virus. The results confirmed that aerosolized cidofovir can provide a barrier to orthopoxvirus infection throughout the respiratory tract.
MATERIALS AND METHODS
Administration of radiolabeled cidofovir.
The 14C-labeled diammonium salt of HPMPC (2-14C) was provided by Gilead Pharmaceuticals (Foster City, Calif.). The initial radiochemical purity of the drug was established at 98.6%, with an estimated decomposition rate of 0.5%/month.
In separate experiments, two groups of weanling female BALB/c mice, averaging 10 g in weight, were given either a single s.c. injection or exposed to aerosolized 14C-labeled cidofovir (14C-cidofovir) dissolved in phosphate-buffered saline (PBS). The target dose for both s.c. and aerosolized exposure was 1 mg/kg (body weight). The methods of injection and aerosol exposure were similar to those previously described (2, 3, 17). Control groups were treated by the same routes with PBS only. For both aerosolized exposure and s.c. injection, a small pilot study was initially performed, followed by a definitive study with a larger number of animals. In both instances, the results of the two experiments were in agreement, and only the results of the larger studies are presented here.
After injection or aerosol exposure, three animals per time point per group were euthanized by an overdose of sodium pentobarbital at 0, 1, 24, 48, 72, and 96 h postexposure. Because the pharmacokinetics of inhaled cidofovir have not previously been studied, three additional time points (3, 6, and 12 h) were added to the aerosolized group to examine the short-term kinetics of the aerosolized drug. Necropsy generally began 30 min before and was completed by 30 min after each designated time point. One or both lungs (one lung for composite samples and two lungs for individual samples), pericardial blood, the liver (whole liver for individual samples and bisected liver for composite samples), the entire stomach, and the kidneys (one kidney for composite samples and both kidneys for individual samples) were removed from each mouse at necropsy. The organs and blood corresponding to a specific mouse or composite were placed into a covered petri dish, and blood was collected in a preweighed crucible. All samples were stored at −20°C until analysis.
Tissue analysis.
For analysis, the thawed samples were each weighed in a porcelain crucible. The 14C was isolated as 14CO2 after combustion by using a model OX600 Harvey Biological Oxidizer. Briefly, crucibles were introduced into a tube furnace at 900°C with an oxygen flow of 350 ml/min and then burned (oxidized) for 4 min. Combustion gases were passed through a catalyst to ensure complete oxidation, and the resultant 14CO2 was trapped in 15 ml of an amine-based scintillation fluid. The trap was removed from the oxidizer, and the scintillation fluid was poured into a scintillation vial for subsequent analysis. The fluid was analyzed by a scintillation counter, and the results were expressed as disintegrations per minute per milligram of tissue. The recovery rate for the biological oxidizer was determined by using cotton balls wetted with 100 μl of [14C]mannitol with or without scintillation fluid; radioactive recovery rates of >99% were consistently obtained.
Intranasal cowpox virus challenge.
Intranasal infection with cowpox Brighton virus and aerosol treatment with nonradiolabeled cidofovir were performed as previously described (2, 3, 17). A dose of ca. 5 × 106 PFU of cowpox virus was applied to the nares of each animal with a micropipette. Aerosol-treated groups received the same “high” and “low” doses of cidofovir used in earlier experiments, which fell within the ranges of 0.5 to 5.0 and 0.06 to 0.5 mg/kg, respectively. As before, groups treated with subcutaneous cidofovir received a dose of 100 mg/kg. Each group was treated once on day −2, −1, 0, +1, or +2, with respect to intranasal viral challenge. A control group received PBS only on day 0.
Lab animal usage.
All research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). The facility where this research was conducted is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
RESULTS
Pharmacokinetics.
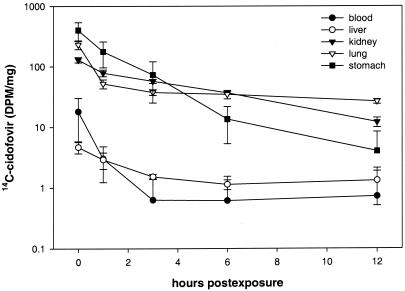
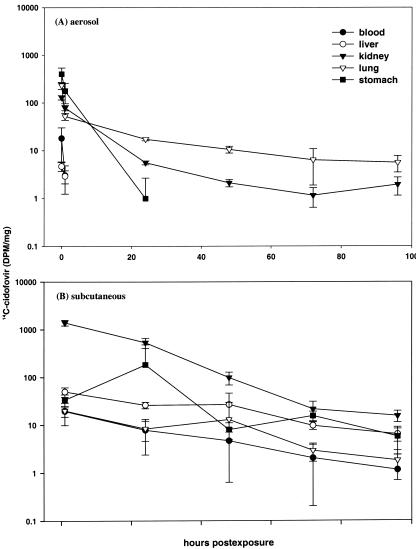
The tissue distribution of radiolabeled cidofovir in aerosol-treated mice during the first 12 h after exposure is shown in Fig. 1 As expected, the highest initial levels were seen in the stomach and lung, representing swallowing and inhalation, respectively, of radiolabeled drug. The concentration in the lung dropped significantly between 0 and 1 h and then leveled off, suggesting a two-compartment (steady-state) pharmacokinetic model. At 1 h postexposure, the concentration of radiolabeled cidofovir in the kidneys was somewhat higher than in the lungs, but the kidney level continued to decrease, whereas the lung concentration remained essentially unchanged over the next 11 h. Over the period from 24 through 96 h postexposure, the cidofovir level in the kidneys continued to decrease more rapidly than in the lungs (Fig. 2A) Drug concentrations in the blood, stomach, and liver fell quickly during the first day postexposure, and only a small residual quantity was detected in the stomach at 24 h.
FIG. 1.
Early-phase pharmacokinetics of 14C-cidofovir administered at 1 mg/kg by inhalation at time point zero (immediately after dosing) and at 1, 3, 6, and 12 h postexposure. Group means ± the standard deviations (n = 4/time point) are shown.
FIG. 2.
Pharmacokinetics of 14C-cidofovir administered at 1 mg/kg either by inhalation (A) or s.c. injection (B). Group means ± the standard deviations (n = 4/time point) are shown.
An s.c. injection of the same target dose of radiolabeled cidofovir (1 mg/kg) produced a strikingly different picture, one in which the drug concentration in the kidneys was much higher than in other tissues at all time points (Fig. 2B) and far higher than the levels observed in aerosol-treated mice. For example, the kidney concentration at 24 h postexposure was ca. 536 dpm/mg in s.c.-inoculated mice but only 6 dpm/mg in aerosolized animals. When these values were expressed in terms of the ratio of lung to kidney concentrations, the level of cidofovir in the lungs of aerosolized mice was found to be roughly threefold higher than in the kidneys at 24 h (Fig. 2A), whereas in the s.c.-inoculated mice the kidney concentration was 65-fold higher than in the lungs (Fig. 2B). Drug concentrations in the tissues of s.c.-treated mice declined gradually over the 96-h time course of the experiment; the fluctuation in drug concentration in the stomach is unexplained.
The striking differences in the relative tissue distributions of cidofovir after aerosol delivery and s.c. injection were made even more obvious when we calculated the percentage of radioactivity recovered at each time point that was represented by the lungs and kidneys (Fig. 3). In mice exposed by aerosol, ca. 60% of the total recovered radioactivity was localized to the lungs at 24 h, and the percentage increased further over time as the kidney concentration declined. In s.c.-inoculated mice, in contrast, almost 90% of the drug had already been partitioned to the kidney by 1 h postexposure, and ca. 30% of the total radioactivity remained there at 96 h. In s.c.-inoculated mice, the percentage of recovered radioactivity that localized to the lungs never exceeded 3%.
FIG. 3.
Pharmacokinetics of 14C-cidofovir administered at 1 mg/kg by s.c. injection (SC) or inhalation (Aerosol) expressed as an organ-specific fraction of the sum total of radioactivity detected in all tissues and blood included in the analysis.
Protection against intranasal cowpox virus challenge.
Treatment with aerosolized cidofovir was successful in protecting against lethal intranasal cowpox infection (Table 1). A dose of drug in the range of 0.5 to 5 mg/kg was protective when given before or after infection; an 80% survival rate was observed when mice were treated 2 days before challenge. A substantially reduced dose (0.06 mg/kg) given by aerosol did not result in a level of protection as long-lived as that resulting from the larger dose. A dose of aerosolized drug of 0.5 to 5 mg/kg resulted in survival similar to that provided by an s.c. dose of 100 mg/kg.
TABLE 1.
Survival of weanling BALB/c mice infected with aerosolized cowpox virus and treated once with aerosolized or s.c.-injected cidofovir on the days indicated relative to the day of infection
| Route of therapy (dose [mg/kg])a | Day of treatment | Survival
|
Pb | |
|---|---|---|---|---|
| No. of animals surviving/total no. of animals | % | |||
| Aero high (0.5-5.0) | −2 | 8/10 | 80 | >0.05 |
| −1 | 9/10 | 90 | >0.05 | |
| 0 | 10/10 | 100 | >0.05 | |
| +1 | 10/10 | 100 | >0.05 | |
| +2 | 9/10 | 90 | >0.05 | |
| Aero low (0.06-0.5) | −2 | 0/10 | 0 | NS |
| −1 | 7/10 | 70 | >0.05 | |
| 0 | 10/10 | 100 | >0.05 | |
| +1 | 9/10 | 90 | >0.05 | |
| +2 | 7/10 | 70 | >0.05 | |
| s.c. (100) | −2 | 7/10 | 70 | >0.05 |
| −1 | 7/10 | 70 | >0.05 | |
| 0 | 10/10 | 100 | >0.05 | |
| +1 | 10/10 | 100 | >0.05 | |
| +2 | 10/10 | 100 | >0.05 | |
| Placebo | 0 | 0/10 | 0 | NS |
Aero high, high aerosolized dose; Aero low, low aerosolized dose.
Significance was determined by Fisher’s exact comparison of cidofovir-treated animals versus placebo-treated animals. NS, not significant.
DISCUSSION
The results of the drug distribution study indicate that aerosolized cidofovir is retained in the respiratory tract and that the pharmacokinetics of cidofovir differ markedly when the drug is administered by aerosol versus when it is given by s.c. injection (4, 5, 6, 7, 24). The data on protection against intranasal cowpox virus challenge provide additional evidence that aerosolized cidofovir could be an effective prophylactic medication for treating orthopoxvirus infections that originate in the human respiratory tract. The drug might be used in a manner similar to aerosolized zanamavir, which significantly decreases the occurrence of influenza when self-administered soon after exposure to ill individuals (11). However, because our mouse model provides only proof-of-concept data, it will be necessary to perform further experiments in nonhuman primates in order to obtain a more realistic assessment of the feasibility of using aerosolized cidofovir to prevent or treat poxvirus infections of the human respiratory tract.
Cidofovir is currently licensed only for the treatment of retinal cytomegalovirus infection in patients with AIDS. The drug must be given by intravenous infusion, accompanied by increased hydration and treatment with probenecid to prevent excessive accumulation in the kidneys (7, 16). Despite the drug's potent activity against variola virus (1), the difficulty of administration and the risk of nephrotoxicity would significantly limit its role in preventing smallpox infection in an outbreak situation. Our study shows that both problems could potentially be avoided by delivering cidofovir directly to the respiratory tract. Our data suggest that solid protection might be obtained by using only a fraction of the dose that would have to be administered intravenously, whereas the drug's retention within the respiratory tract would prevent its accumulation to toxic levels in the kidneys.
Self-administered aerosolized cidofovir would clearly be valuable in a setting of imminent or recent exposure to aerosolized variola virus. At present, plans for the response to a smallpox outbreak call for the immediate vaccination of all exposed individuals (13). It would therefore be important to ensure that simultaneous treatment with aerosolized cidofovir would not interfere with the development of a vaccination lesion and the resulting immune response. Our data suggest that aerosolized cidofovir would not have a significant effect on vaccination, since delivery of the drug directly to the respiratory tract would result in negligible distribution to other tissues, including the skin. It is thus reasonable to propose a dual approach to smallpox prophylaxis: the administration of aerosolized cidofovir, to provide immediate short-term protection, plus vaccination, to provide long-term immunity.
In addition to its role in preventing smallpox infection, aerosolized cidofovir might also have a useful role in reducing transmission of virus from those already infected. The delivery of drug to tissues lining the airways could prevent the development of the oropharyngeal enanthem that is the source of aerosolized virus in person-to-person transmission (10). Such therapy could reduce the risk of exposure to healthcare workers and blunt the development of a smallpox epidemic.
In this and previous studies, mice received a whole-body exposure to aerosolized drug and virus from an aerosol generator producing a particle size of ca. 1 μm. Approximately 50% of the particles of this size are deposited in the lungs of mice by this exposure modality, with fractions of the remaining particles deposited in the bronchiolar and nasopharnygeal regions of the respiratory tract (19). Oral uptake is minimal with a particle of this size; <5% of a mouse's inhaled dose is composed of particles that end up in the gut (19). The fractional deposition pattern observed in previous studies with the same size aerosol may explain the protective efficacy observed in the intranasal challenge model. In contrast to mice, human self-administration of cidofovir should incorporate methodology that delivers the widest dissemination throughout the respiratory tract to ensure protection against infectious particles of various sizes.
Current concern about a terrorist introduction of smallpox is further heightened by the awareness that biotechnology now makes possible the deliberate modification of orthopoxviruses to increase their virulence. A recent report demonstrated that the virulence of ectromelia (mousepox) virus for mice could be markedly increased by introducing the gene for murine interleukin-4 into the viral genome (15). It is possible that more virulent versions of variola virus might be created in a similar manner. However, although an orthopoxvirus might be modified to overcome immunological barriers to dissemination, current evidence indicates that these viruses cannot readily be made resistant to DNA polymerase inhibitors, such as cidofovir. The sequence of the polymerase drug-binding site is highly conserved among orthopoxvirus species, indicating that mutations in that region are deleterious to viral replication (R. Baker, unpublished data). In addition, efforts to select for cidofovir-resistant mutants have shown that the acquisition of drug resistance is accompanied by a decrease in virulence (22). The findings suggest that cidofovir would provide an effective defense even against genetically modified orthopoxviruses.
Acknowledgments
We thank Debbie Kefauver, Josh Shamblin, and Michael West for their technical expertise in this project.
This study was sponsored by the Medical Biological Defense Research Program, U.S. Army Medical Research and Materiel Command, project number 02-4-41-090, and by U.S. Army Medical Research Institute of Infectious Diseases external contract number DAMD17-01-P-1000.
The opinions, interpretations, conclusions, and recommendations expressed here are those of the authors and are not necessarily endorsed by the U.S. Army.
REFERENCES
- 1.Baker, R., M. Bray, and J. Huggins. 2003. Potential antiviral therapeutics for smallpox and other orthopoxvirus infections. Antiviral Res. 57:13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray, M., M. Martinez, D. Kefauver, M. West, and C. Roy. 2002. Treatment of aerosolized cowpox virus infection in mice with aerosolized cidofovir. Antivir. Res. 54:129-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray, M., M. Martinez, D. Smee, D. Kefauver, E. Thompson, and J. Huggins. 2000. Cidofovir protects mice against lethal aerosol or intranasal cowpox virus challenge. J. Infect. Dis. 181:10-19. [DOI] [PubMed] [Google Scholar]
- 4.Cundy, K. C., Z. H. Li, M. J. Hitchcock, and W. A. Lee. 1996. Pharmacokinetics of cidofovir in monkeys: evidence for a prolonged elimination phase representing phosphorylated drug. Drug Metab. Dispos. 24:738-744. [PubMed] [Google Scholar]
- 5.Cundy, K. C., G. Lynch, and W. A. Lee. 1997. Bioavailability and metabolism of cidofovir following topical administration to rabbits. Antivir. Res. 35:113-122. [DOI] [PubMed] [Google Scholar]
- 6.Cundy, K. C. 1999. Clinical pharmacokinetics of the antiviral nucleotide analogues cidofovir and adefovir. Clin. Pharmacokinet. 36:127-143. [DOI] [PubMed] [Google Scholar]
- 7.Cundy, K. C., A. Bidgoo, G. Lynch, J. Shaw, L. Griffin, and W. A. Lee. 1996. Pharmacokinetics, bioavailability, metabolism and tissue distribution of cidofovir (HPMPC) and cyclic HPMPC in rats. Drug Metab. Dispos. 24:745-752. [PubMed] [Google Scholar]
- 8.De Clercq, E. 2002. Cidofovir in the treatment of poxvirus infections. Antivir. Res. 55:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Clercq, E. 2002. Cidofovir in the therapy and short-term prophylaxis of poxvirus infections. Trends Pharmacol. Sci. 23:456-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fenner, F., D. A. Henderson, I. Arita, Z. Jezek, and I. D. Ladnyi. 1988. Smallpox and its eradication. World Health Organization, Geneva, Switzerland.
- 11.Hayden, F., L. Gubareva, A. Monto, et al. 2000. Inhaled zanamavir for the prevention of influenza in families. N. Engl. J. Med. 343:1282-1289. [DOI] [PubMed] [Google Scholar]
- 12.Henderson, D. A. 1998. Bioterrorism as a public health threat. 1998. Emerg. Infect. Dis. 4:488-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson, D. A. 1999. Smallpox as a biological weapon: medical and public health management. JAMA 281:2127-2137. [DOI] [PubMed] [Google Scholar]
- 14.Ho, H. T., K. Woods, J. Bronson, H. De Boeck, J. Martin, and M. Hitchcock. 1992. Intracellular metabolism of the antiherpes agent (S)-1-[3-hydroxy-2-(phosphonylmethoxy)propyl]cytosine. Mol. Pharmacol. 41:197-202. [PubMed] [Google Scholar]
- 15.Jackson, R. J., A. Ramsay, C. Christensen, S. Beaton, D. Hall, and I. Ramshaw. 2001. Expression of mouse interleukin-4 by a recombinant ectromelia virus suppresses cytolytic lymphocyte responses and overcomes genetic resistance to mousepox. J. Virol. 75:1205-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lacy, S. A., M. J. Hitchcock, W. A. Lee, P. Tellier, and K. C. Cundy. 1998. Effect of oral probenecid coadministration on the pharmacokinetics of intravenous cidofovir in cynomolgus monkeys. Toxicol. Sci. 44:97-106. [DOI] [PubMed] [Google Scholar]
- 17.Martinez, M. J., M. Bray, and J. Huggins. 2000. A mouse model of aerosol-transmitted orthopoxviral disease: morphology of experimental aerosol-transmitted orthopoxviral disease in a cowpox virus-BALB/c mouse system. Arch. Pathol. Lab. Med. 124:362-377. [DOI] [PubMed] [Google Scholar]
- 18.Naessens, L., R. Snoeck, G. Andrei, J. Balzarini, J. Neyts, and E. de Clercq. 1997. HPMPC (cidofovir), PMEA(adefovir), and related acyclic nucleoside phosphonate analogues: a review of their pharmacology and clinical potential in the treatment of viral infections. Antivir. Chem. Chemother. 8:1-23. [Google Scholar]
- 19.Roy, C. J., J. M. Hartings, M. Hale, S. Duniho, and L. M. Pitt. 2003. Impact of inhalation exposure modality and particle size on the respiratory deposition of ricin in BALB/c mice. Inhal. Toxicol. 15:619-628. [DOI] [PubMed] [Google Scholar]
- 20.Smee, D. F., K. W. Bailey, M. H. Wong, and R. W. Sidwell. 2001. Effects of cidofovir on the pathogenenesis of a lethal vaccinia virus respiratory infection in mice. Antivir. Res. 52:55-62. [DOI] [PubMed] [Google Scholar]
- 21.Smee, D. F., K. W. Bailey, M. Wong, and R. W. Sidwell. 2000. Intranasal treatment of cowpox virus respiratory infections in mice with cidofovir. Antivir. Res. 47:171-177. [DOI] [PubMed] [Google Scholar]
- 22.Smee, D. F., R. Sidwell, D. Kefauver, M. Bray, and J. Huggins. 2002. Characterization of wild-type and cidofovir-resistant strains of camelpox, cowpox, monkeypox, and vaccinia viruses. Antimicrob. Agents Chemother. 46:1329-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snoeck, R., and E. de Clercq. 2002. Role of cidofovir in the treatment of DNA virus infections, other than CMV infections, in immunocompromised patients. Curr. Opin. Investig. Drugs 3:1561-1566. [PubMed] [Google Scholar]
- 24.Wachsman, M., B. Petty, K. Cundy, H. Jaffe, P. Fisher, A. Pastelak, and P. Lietman. 1996. Pharmacokinetics, safety, and bioavailability of HPMPC (cidofovir) in human immunodeficiency virus-infected subjects. Antivir. Res. 29:153-161. [DOI] [PubMed] [Google Scholar]
- 25.Wehrle, P. F., P. Posch, K. Richter, and D. A. Henderson. 1970. An air-borne outbreak of smallpox in a German hospital and its significance with respect to other recent outbreaks in Europe. Bull. W. H. O. 43:669-679. [PMC free article] [PubMed] [Google Scholar]