Abstract
Between 1993 and 1999, we monitored the efficacy of sulfadoxine-pyrimethamine in 1,175 children aged <24 months receiving 2,789 treatments for falciparum malaria in western Kenya using a widely deployed age-based dose regimen: infants, 125 plus 6.25 mg (sulfadoxine plus pyrimethamine); children aged 12 to 23 months; 250 plus 12.5 mg. Cumulative treatment failure by day 7, defined as early clinical failure by day 3 or presence of parasitemia on day 7, increased from 18% in 1993 to 1994 to 22% in 1997 to 1998 (P-trend test = 0.20). Based on body weight, the median dose received was 20 plus 1.00 mg/kg, and 73% of the treatments were given at lower than the recommended target dose of 25 plus 1.25 mg/kg. Underdosing accounted for 26% of cumulative treatment failures. After the dose was increased in 1998 (median, 36 plus 1.8 mg/kg), only 4.2% of patients received less than 25 plus 1.25 mg/kg and there was no association with treatment failure. However, the proportion of cumulative treatment failure continued to increase to 27% by 1999 (P-trend test = 0.03). These results raise concern about the longevity of sulfadoxine-pyrimethamine in these settings. Underdosing may have contributed to the rate at which sulfadoxine-pyrimethamine resistance developed in this area. Treatment guidelines should ensure that adequate doses are given from the initial deployment of antimalarials onward.
In the last decade several countries in sub-Saharan Africa have switched or are considering switching first-line treatment of uncomplicated falciparum malaria from chloroquine to sulfadoxine-pyrimethamine (S-P), alone or in combination with other antimalarials. Close monitoring of S-P efficacy will be necessary to assess its durability.
We previously monitored the efficacy of S-P from 1993 to 1997 in children younger than 5 years with acute, nonsevere falciparum malaria in an area of western Kenya with intense malaria transmission. The dose of S-P, which was based on age, was found to be an important predictor of treatment efficacy, explaining a quarter of all treatment failures by day 7 (11). Based on their body weight at the time of treatment, three-quarters of the children received less than the internationally recommended target dose of 25 plus 1.25 mg of S-P (25 mg of sulfadoxine plus 1.25 mg of pyrimethamine) per kg of body weight. Similar age-based S-P dose regimens are widely deployed in sub-Saharan Africa. Underdosing also provides an opportunity for the selection of resistant mutants and has been suggested to play a role in the development of resistance (3). These findings led to a revision of the dose recommendations by the Kenyan Ministry of Health in 1998 (2). Here we describe the results of continued annual surveillance of S-P efficacy at the same study site after the introduction of a new dose regimen for S-P in April 1998 that aimed to reduce the proportion of children receiving less than the target dose of 25 plus 1.25 mg of S-P per kg of body weight. The results are compared to the risk of failure before April 1998.
MATERIALS AND METHODS
Procedures.
The site and method have been described in detail previously (1, 10, 11). In short; this treatment study was part of the Asembo Bay Cohort Project, a prospective study on the acquisition of natural immunity to malaria (1). Children enrolled in the Asembo Bay Cohort Project, aged less than 5 years (April 1993 to April 1998) or less than 2 years (April 1998 to April 1999) with microscopically confirmed nonsevere falciparum malaria, received a supervised dose of S-P: infants, 125 plus 6.25 mg; children aged 12 to 23 months, 250 plus 12.5 mg. Home follow-up occurred on day 2 or 3 and on day 7, with revisits on day 4 or 8 to 10 if the participant was not at home on the scheduled days. Previous analysis indicated that children with a documented history of S-P intake within the previous month were at a 1.7-fold increased risk of subsequent treatment failure when retreated with S-P (11). Recent S-P use (within 28 days) was therefore introduced as a contraindication for S-P treatment from April 1998 onward. All treatment (Table 1) was given under supervision of study staff, and the quality of S-P was confirmed by high-performance liquid chromatography.
TABLE 1.
Characteristics of children and treatment regimens used
| Characteristic | Apr 1993-Mar 1998 (n = 2,496) | Apr 1998-Mar 1999 (n = 293) |
|---|---|---|
| S-P dose regimen in children aged: | ||
| 0-1 mo | 125+6.25 mg (1/4 tablet) | 125+6.25 mg (1/4 tablet) |
| 2-11 mo | 125+6.25 mg (1/4 tablet) | 250+12.5 mg (1/2 tablet) |
| 12-23 mo | 250+12.5 mg (1/2 tablet) | 375+18.75 mg (3/4 tablet) |
| Pyrimethamine dose (mg/kg), median (IQRd) | 1.00 (0.79-1.26) | 1.79 (1.58-2.08) |
| % < 25 plus 1.25 mg/kga | 72.6 | 4.2 |
| % < 27.5 plus 1.375 mg/kgb | 84.9 | 10.6 |
| Age (mo), median (IQR) | 10.1 (6.1-15.4) | 10.3 (5.9-14.3) |
| Parasite density, median (IQR) | 9,533 (3,000-22,120) | 10,240 (5,653-19,893) |
| History of chloroquine in past 2 wk, no. (%) | 485 (19.4) | 46 (15.7) |
| HbAS phenotype, no. (%)c | 349 (15.5) | 63 (23.3) |
Internationally recommended target dose of 25 plus 1.25 mg/kg.
Most discriminative dose which differentiates between treatment failure and success.
Phenotype missing for 240 treatments before 1 April 1998 and 22 after 1 April 1998.
IQR, interquartile range.
Definitions.
Treatment failures and successes were defined using a modified version of the World Health Organization classification system (11, 16). Early clinical failure was defined as either clinical deterioration requiring alternative treatment or persistence of fever on day 2 with pure or mixed falciparum parasitemia that was greater than the pretreatment density or persistence of any Plasmodium falciparum parasites with fever by day 3 or 4. Parasitological treatment failure was defined as the presence of P. falciparum parasites on days 7 to 10 (referred to as day-7), regardless of the presence of symptoms, or a percent decline in parasitemia by day 2 or 3. Cumulative treatment failure by day 7 was defined as having either an early clinical failure by days 2 to 4 or a parasitological treatment failure by days 7 to 10.
Statistical analysis.
The SAS software packages SUDAAN release 8 and SAS version 8.0 (SAS, Inc., Research Triangle Institute, Research Triangle Park, N.C.) were used. Confidence intervals (CI) and P values were corrected for multiple observations per child. The effect of treatment dose on parasitological failure by day 7 was modeled by using multivariate log-binomial regression analysis, adjusting for age and study year, with treatment dose categorized in five groups of 5-/0.25-mg/kg S-P and entered as a nominal variable (Fig. 1). P-trend values were obtained from similar models by entering the treatment dose as an ordinal variable, using the same categories. Previous comparison of the sensitivity, specificity, and Youden index scores indicated that 27.5 plus 1.375 mg/kg was the most discriminative threshold concentration, below which an increased risk of treatment failure was observed in children younger than 5 years (11). To define the proportion of treatment failures that could be attributed to treatment doses below this concentration, the attributable risk (or population attributable fraction) was calculated. This was done by calculating [prevalence × (RR − 1)]/ {1 + [prevalence × (RR − 1)]} where RR denotes relative risk and prevalence denotes the proportion of children that received doses lower than 27.5 plus 1.375 mg/kg. For all statistical tests, a two-sided P < 0.05 was considered to be significant.
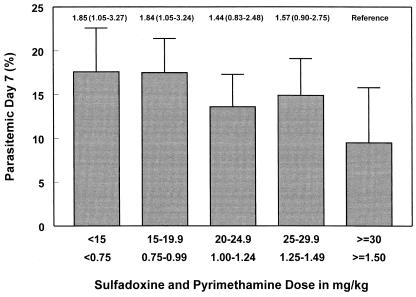
FIG. 1.
Relationship between treatment dose (in milligrams of S-P received per kilogram of body weight) and the probability of being parasitemic 7 days after treatment between April 1993 and April 1998. Error bars represent the upper 95% CI, corrected for multiple observations per child. Values above the error bars represent the relative risk (95% CI) of treatment failure, with the highest-dose group as the reference. The S-P dose used was based on age: 125 plus 6.25 mg (one-quarter of a tablet) in infants, and 250 plus 12.5 mg (one-half of a tablet) in 12- to 23-month-old children (17).
The effects of time on treatment outcome were modeled using log-binomial regression, with the study year entered as an ordinal variable in categories of 2 years and adjusting for age, treatment dose, baseline parasite density, degree of fever, and HbAS phenotype (Fig. 2) (10, 11). Other covariates examined but not found to be related to the treatment failure risk were bednet randomization group (from January 1997 onward) (9) and season. To allow for a longitudinal comparison over time, only children younger than 2 years and without a history of S-P use in the 4-week period before enrolment were included in this analysis.
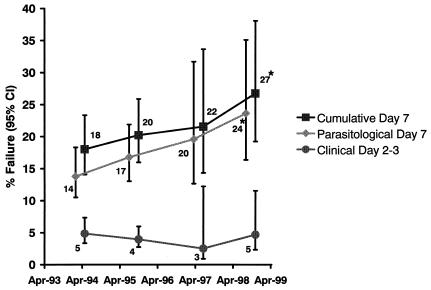
FIG. 2.
Proportion of children younger than 2 years with early clinical failure by days 2 to 3 (bottom line), parasitological treatment failure on day 7 (middle line), or either early clinical failure or parasitological treatment failure by day 7 (cumulative treatment failure; top line). Each point on the graph represents the failure risk at the median date for each time category (April 1993 to December 1994, January 1995 to December 1996, January 1997 to March 1998, and April 1998 to March 1999). Error bars represent the 95% CI and were corrected for multiple observations per child. Asterisks indicate a statistically significant increase in treatment failure over time (P-trend test). The S-P dose used was based on age: 125 plus 6.25 mg (one-quarter of a tablet) in infants and 250 plus 12.5 mg (one-half of tablet) in 12- to 23-month-old children (17). In April 1998, the dose was increased to 125 plus 6.25 mg (one-quarter of a tablet), 250 plus 12.5 mg (one-half of a tablet), and 375 plus 18.75 mg (three-quarters of a tablet) for children aged 0 to 2, 2 to 11, and 12 to 23 months, respectively.
Informed consent.
This study was approved by the institutional review boards of the Kenya Medical Research Institute and the Centers for Disease Control and Prevention, Atlanta, Ga. Informed consent was obtained from all caregivers after explanation of the study procedures in the local language.
RESULTS
Between April 1993 and April 1998, 991 children younger than 2 years received 2,496 treatments with S-P. After the April 1998 dose increase through April 1999, a further 293 treatments of 200 children were followed until day-7 (Table 1). Follow-up data were available for 79.0% of treatments by days 2 to 3 and for 75.0% by day-7. The overall proportion of early clinical failures by days 2 to 3 was 4.4% and remained stable throughout the study period, but the parasitological failure risk by day-7 increased from 14% in 1993 to 1994 to 20% in 1997 to 1998 (Fig. 2). Before April 1998, 73% of children younger than 2 years (76% of children younger than 5 years) had received <25 plus 1.25 mg/kg (Table 1). There was an inverse relationship between the S-P dose (in milligrams per kilogram) received and the probability of parasitological treatment failure by day 7 (P-trend test = 0.046, adjusted for age and year [Fig. 1]). The relative risk associated with the most discriminative dose below which an increased risk of parasitological treatment failure was observed (27.5 plus 1.375 mg/kg) (11) was 1.41 (95% CI, 1.01 to 1.96; P = 0.045, adjusted for age and study year). Eighty-five percent of treatments between 1993 and 1998 resulted in doses below this threshold. The corresponding proportions after the increase in the dose were 4.2% (<25 plus 1.25 mg/kg) and 10.6% (<27.5 plus 1.375 mg/kg) (Table 1). Between 1993 and 1998, the attributable risk for parasitological treatment failure associated with doses below 27.5 plus 1.375 mg/kg was 26.3% (95% CI, 0.8 to 45.4). There was no relationship between dose and treatment failure after the dose increase. Nevertheless a continued increase in treatment failure risk was observed; between April 1998 and April 1999, 27% of treatments failed by day 7 (Fig. 2).
DISCUSSION
This study observed an increase in parasitological treatment failure with S-P treatment within 5 years of its introduction as first-line treatment for uncomplicated falciparum malaria in young children in this study area. The high failure risk observed is consistent with recent reports from a treatment trial in northeast Tanzania (5). Although there was no evidence of increasing high-grade clinical resistance in our study, close monitoring of the children in the Tanzanian study showed that two-thirds of children who failed to clear their parasites by day-7 developed clinical malaria within 4 weeks (5). Failure risk assessed by day-7 is likely to underestimate the degree of resistance in the population, because with slowly eliminated drugs such as S-P, most recrudescences in patients in areas with predominantly low-grade resistance occur more than 14 days after treatment. This is the period required to reach patent parasitemia from low parasite levels with multiplication rates being suppressed by host defenses and residual inhibitory concentrations of the drug (14). These results raise concern about the longevity of S-P as monotherapy in these settings.
Between 1993 and 1998, approximately 50% of the population younger than 5 years was enrolled in the Asembo Bay Cohort Project and received S-P as first-line treatment for symptomatic nonsevere falciparum malaria. Although S-P had been introduced into Kenya as a second-line treatment in 1983, it was not widely available in the study area outside the research setting and accounted for less than 1% of S-P treatments reported by caregivers between 1993 and 1997 (10). This changed by early 1999, when the Kenyan Ministry of Health introduced S-P as the new first-line treatment for uncomplicated malaria in this part of Kenya (2). Thus, most of the increase in parasitological treatment failures for S-P in this area occurred prior to its introduction on a larger scale. Of note also is the relatively high failure risk of 18% in 1993.
Apart from innate resistance of the parasite (4), a variety of other factors can contribute to treatment failure in individual patients, including high initial parasite biomass, low host immunity, and insufficient drug concentrations (15). Under these controlled research settings, the intake of S-P was observed and retreatment took place when vomiting occurred. The quality of the study drug used was confirmed, and parasite densities were within the normal range for young children with mild symptomatic falciparum malaria; these factors are unlikely to explain the observations. The present study suggests that a high proportion of treatment failures could be attributed to the relatively low treatment dose (in milligrams per kilogram of body weight) received by most children. Based on the age-based dose regimen used before April 1998, three-quarters of the children received less than 25 plus 1.25 mg/kg. Doses below 27.5 plus 1.375 mg/kg, the most discriminative threshold concentration associated with treatment failure in previous analyses (11), explained one-quarter of parasitological treatment failures by day 7. An increase in the dose in April 1998 markedly reduced the proportion of underdosing, but this did not result in a decrease in treatment failures, and the rate of annual increase in failure risk remained unchanged (Fig. 2).
Mathematical modeling based on in vitro and in vivo data for mefloquine (as monotherapy) suggest that initial deployment of lower doses provides an opportunity for the selection of resistant mutants and would be expected to lead more rapidly to resistance than the de novo use of maximal doses (8). Similar considerations are likely to apply to S-P. Resistance to S-P arises from point mutations in the plasmodial dihydrofolate reductase (dhfr) and dihydropteroate synthetase (dhps) genes (7). The use of S-P is associated with a strong pressure for the selection of resistant alleles (6, 13), which is particularly likely to occur when parasites encounter submaximal concentrations of S-P in blood (3), resulting in persistence and preferential transmission of S-P-resistant parasites. Many young children, such as in our study, have insufficient host immunity to clear these resistant infections (3, 8). Furthermore, S-P is known to stimulate infective gametocytemia more than other antimalarials do (12). Thus, the low treatment dose used in the first 5 years of the study is likely to have contributed to the rate at which the initial S-P resistance was able to develop in this population. The previous study of young children from northeastern Tanzania showed that parasitological treatment failure by day 7 following adequate treatment doses of S-P (≥25 plus 1.25 mg/kg) was due primarily to parasites with three mutations in the dhfr domain (S108N, N51I, and C59R) (5). The existence of these triple-mutant parasites in our study site may explain the lack of a significant effect on treatment failure risk after the dose was increased in 1998.
The age-based dose regimen used until 1998 is still recommended by the majority of medical textbooks in 2003 (11). Underdosing is one of the few controllable determinants of the rate at which antimalarial drug resistance develops. It is critical to ensure that treatment guidelines result in adequate doses at the initial deployment of antimalarials when countries alter first-line treatment for nonsevere falciparum malaria. This is particularly relevant when drugs are deployed as monotherapy and are not protected by the concomitant administration of a companion drug with a different mode of action and resistance (combination therapy). For areas such as western Kenya and northeastern Tanzania, it is time to begin to reconsider the role of S-P prior to the emergence of high levels of clinical failure.
Acknowledgments
We thank the parents and guardians of the children who participated in the study and the many people who assisted with this project. We thank the Director of the Kenya Medical Research Institute for his permission to publish this work. We thank Lawrence Slutsker and Richard Steketee from CDC for critical review of the manuscript.
Dianne Terlouw and Feiko ter Kuile were partially supported by grants from The Netherlands Foundation for the Advancement of Tropical Research, The Hague, The Netherlands (WOTRO; grants W93-323 and W93-273). The Asembo Bay Cohort Project was funded by the U.S. Agency for International Development (grant HRN-60010-A-00-4010-00).
The opinions or assertions contained in this paper are the private ones of the authors and are not to be construed as official or reflecting the views of the U.S. Public Health Service or Department of Health and Human Services. Use of trade names is for identification only and does not imply endorsement by U.S. Public Health Service or Department of Health and Human Services.
Footnotes
Asembo Bay Cohort Project manuscript XIX.
REFERENCES
- 1.Bloland, P. B., T. K. Ruebush, J. B. McCormick, J. Ayisi, D. A. Boriga, A. J. Oloo, R. Beach, W. Hawley, A. Lal, B. Nahlen, V. Udhayakumar, and C. C. Campbell. 1999. Longitudinal cohort study of the epidemiology of malaria infections in an area of intense malaria transmission. I. Description of study site, general methodology, and study population. Am. J. Trop. Med. Hyg. 60:635-640. [DOI] [PubMed] [Google Scholar]
- 2.Kenya Ministry of Health. 1998. National guidelines for diagnosis, treatment & prevention of malaria for health workers. Kenya Ministry of Health, Nairobi.
- 3.Kun, J. F., L. G. Lehman, B. Lell, R. Schmidt-Ott, and P. G. Kremsner. 1999. Low-dose treatment with sulfadoxine-pyrimethamine combinations selects for drug-resistant Plasmodium falciparum strains. Antimicrob. Agents Chemother. 43:2205-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendez, F., A. Munoz, G. Carrasquilla, D. Jurado, M. Arevalo-Herrera, J. F. Cortese, and C. V. Plowe. 2002. Determinants of treatment response to sulfadoxine-pyrimethamine and subsequent transmission potential in falciparum malaria. Am. J. Epidemiol. 156:230-238. [DOI] [PubMed] [Google Scholar]
- 5.Mutabingwa, T., A. Nzila, E. Mberu, E. Nduati, P. Winstanley, E. Hills, and W. Watkins. 2001. Chlorproguanil-dapsone for treatment of drug-resistant falciparum malaria in Tanzania. Lancet 358:1218-1223. [DOI] [PubMed] [Google Scholar]
- 6.Nzila, A. M., E. Nduati, E. K. Mberu, C. Hopkins Sibley, S. A. Monks, P. A. Winstanley, and W. M. Watkins. 2000. Molecular evidence of greater selective pressure for drug resistance exerted by the long-acting antifolate pyrimethamine/sulfadoxine compared with the shorter-acting chlorproguanil/dapsone on Keriyan Plasmodium falciparum. J. Infect. Dis. 181:2023-2028. [DOI] [PubMed] [Google Scholar]
- 7.Plowe, C. V., J. F. Cortese, A. Djimde, O. C. Nwanyanwu, W. M. Watkins, P. A. Winstanley, J. G. Estrada-Franco, R. E. Mollinedo, J. C. Avila, J. L. Cespedes, D. Carter, and O. K. Doumbo. 1997. Mutations in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase and epidemiologic patterns of pyrimethamine-sulfadoxine use and resistance. J. Infect. Dis. 176:1590-1596. [DOI] [PubMed] [Google Scholar]
- 8.Simpson, J. A., E. R. Watkins, R. N. Price, L. Aarons, D. E. Kyle, and N. J. White. 2000. Mcfloquine pharmacokinetic-pharmacodynamic models: implications for dosing and resistance. Antimicrob. Agents Chemother. 44:3414-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ter Kuile, F. O., D. J. Terlouw, S. K. Kariuki, P. A. Phillips-Howard, L. B. Mirel, W. A. Hawley, J. F. Friedman, Y. P. Shi, M. S. Kolczak, A. A. Lal, J. M. Vulule, and B. L. Nahlen. 2003. Impact of permethrin-treated bed nets on malaria, anemia, and growth in infants in an area of intense perennial malaria transmission in western Kenya. Am. J. Trop. Med. Hyg. 68:68-77. [PubMed] [Google Scholar]
- 10.Terlouw, D. J., M. A. Aidoo, V. Udhayakumar, M. S. Kolczak, A. J. Oloo, P. A. Kager, A. A. Lal, B. L. Nahlen, and F. O. ter Kuile. 2002. Increased efficacy of sulfadoxine-pyrimethamine in the treatment of uncomplicated falciparum malaria among children with sickle cell trait in western Kenya. J. Infect. Dis. 186:1661-1668. [DOI] [PubMed] [Google Scholar]
- 11.Terlouw, D. J., J. M. Courval, M. S. Kolczak, O. S. Rosenberg, A. J. Oloo, P. A. Kager, A. A. Lal, B. L. Nahlen, and F. O. ter Kuile. 2003. Treatment history and treatment dose are important determinants of sulphadoxine-pyrimethamine efficacy in children with uncomplicated malaria in western Kenya. J. Infect. Dis. 187:467-476. [DOI] [PubMed] [Google Scholar]
- 12.von Seidlein, L., C. Drakeley, B. Greenwood, G. Walraven, and G. Targett. 2001. Risk factors for gametocyte carriage in Gambian children. Am. J. Trop. Med. Hyg. 65:523-527. [DOI] [PubMed] [Google Scholar]
- 13.Watkins, W. M., and M. Mosobo. 1993. Treatment of Plasmodium falciparum malaria with pyrimethamine-sulfadoxine: selective pressure for resistance is a function of long elimination half-life. Trans. R. Soc. Trop. Med. Hyg. 87:75-78. [DOI] [PubMed] [Google Scholar]
- 14.White, N. 2002. The assessment of antimalarial drug efficacy. Trends Parasitol. 18:458. [DOI] [PubMed] [Google Scholar]
- 15.White, N. J. 1998. Why is it that antimalarial drug treatments do not always work? Ann. Trop. Med. Parasitol. 92:449-458. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization 1996. Assessment of therapeutic efficacy of antimalarial drugs for uncomplicated malaria in areas with intense transmission. WHO/MAL/96.1077. World Health Organization, Geneva, Switzerland.
- 17.Zucker, J. R., and C. C. Campbell. 1993. Malaria. Principles of prevention and treatment. Infect. Dis. Clin. North Am. 7:547-567. [PubMed] [Google Scholar]