Abstract
The purpose of this study was to compare the activity of HMR 1043 with those of daptomycin and teicoplanin against gram-positive isolates. Susceptibility tests were performed for 52 strains, 26 parental strains, including staphylococcal, streptococcal, enterococcal, and listerial strains, and 26 HMR 1043-resistant mutants obtained from parental strains by using the Szybalski method. Agar dilution and disk diffusion susceptibility tests were performed by the procedures outlined by the NCCLS. HMR 1043 demonstrated good activity against susceptible and resistant gram-positive bacteria. The activity of HMR 1043 in vitro was less influenced by the presence of calcium ions than that of daptomycin. Susceptibility test breakpoints were not defined because of the poor correlation coefficients obtained with the different disks tested.
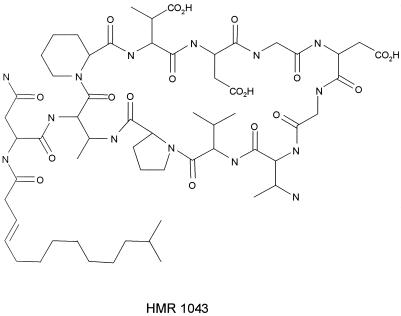
HMR 1043 is a new antibiotic belonging to the class of cyclic lipopeptides that includes daptomycin (6). The structure of HMR 1043 is shown in Fig. 1. These drugs are being developed for the treatment of severe infections due to antibiotic-resistant gram-positive cocci. Daptomycin is active against susceptible and resistant gram-positive isolates, such as vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus, glycopeptide-intermediate staphylococci, and penicillin-resistant pneumococci (2, 4, 8, 11, 12). The antibacterial activity of daptomycin requires the presence of calcium cations. Previous studies have recommended a calcium supplementation of 50 mg/liter for broth microdilution susceptibility tests (1, 5, 7) and of 9 mM (200 mg/liter) for agar dilution susceptibility tests (3).
FIG. 1.
Structure of HMR 1043.
The objective of this study was to compare the activity of HMR 1043 with those of daptomycin and teicoplanin against susceptible and resistant gram-positive isolates, to investigate the effect of calcium supplementation, and to interpret the zone size obtained with different HMR 1043 disks. The Szybalski method (13) was used for the preliminary selection of mutants, because of the rarity of truly resistant gram-positive strains.
The 26 parental strains included 18 clinical and 8 reference isolates: 5 Staphylococcus aureus strains (including 1 with intermediate resistance to teicoplanin and 1 vancomycin-intermediate Staphylococcus aureus strain), 4 Staphylococcus epidermidis strains (including 1 resistant to teicoplanin), 2 Staphylococcus haemolyticus strains (including 1 resistant to teicoplanin), 3 Enterococcus faecalis strains (1 possessing the vanA gene and 1 possessing the vanB gene), 3 Enterococcus faecium strains (1 with the vanA gene and 1 with the vanB gene), 2 Listeria monocytogenes strains, 3 Streptococcus pneumoniae strains (including 1 penicillin resistant), 2 Streptococcus pyogenes strains, and 2 Streptococcus agalactiae strains.
HMR 1043-resistant mutants were selected by plating 109 CFU of overnight cultures of the 26 parental strains grown in Mueller-Hinton (MH) medium on MH agar containing an HMR 1043 gradient up to 32 times the MIC (13). Spontaneously resistant colonies appeared after incubation at 37°C for 24 to 48 h. The colony grown in the highest drug concentration was taken as the mutant strain. Susceptibility tests were performed for the 52 strains, the 26 parental strains and 26 mutants.
HMR 1043 and teicoplanin were kindly provided by Aventis (Romainville, France), and daptomycin was provided by Lilly Research Laboratories (Indianapolis, Ind.). HMR 1043, daptomycin, and teicoplanin concentrations ranged from 0.0038 to 16 μg/ml. Disks with 7.5, 15, 30, 60, and 120 μg of HMR 1043 were prepared. Agar dilution and disk diffusion susceptibility tests were performed by the procedures outlined by the National Committee for Clinical Laboratory Standards (9, 10), with MH agar supplemented with 5% whole sheep blood when streptococcal and listerial strains were tested. The following media were used: base MH agar containing 100 mg of Ca2+ per liter and MH agar supplemented with up to 207 mg (9 mM) of Ca2+ per liter (CSMH) from a 20-g/liter CaCl2 solution. MICs and inhibition zone diameters were read after 18 h of incubation at 37°C.
Comparative antimicrobial activity.
The comparative MICs of HMR 1043, daptomycin, and teicoplanin are shown in Table 1. The activity of HMR 1043 was not influenced by the resistance of staphylococci to methicillin or teicoplanin, the resistance of pneumococci to penicillin, or the resistance of enterococci to vancomycin (vanA or vanB type). HMR 1043 was two- to eightfold less active than daptomycin against listerial isolates. Mutants selected from staphylococci intermediate or resistant to teicoplanin were 4- to 16-fold less susceptible than the parental strains (HMR 1043 MICs, 4 to 8 μg/ml). MICs of daptomycin for parental and mutant streptococcal and listerial strains were identical. Mutants selected from the Staphylococcus epidermidis clinical strains were twofold to eightfold more resistant than the parental strains, with daptomycin MICs of ≤2 μg/ml; MICs of teicoplanin for parental and mutant strains were comparable. Streptococci were more susceptible to teicoplanin than staphylococci.
TABLE 1.
In vitro activity of HMR 1043 and other agents on parental and mutant strainsa
| Organism | Strain | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|---|
| HMR 1043
|
Daptomycin
|
Teicoplanin in MH | ||||
| MH | CSMH | MH | CSMH | |||
| Staphylococcus aureus | ||||||
| Methicillin susceptible ATCC 25923 | 1 | 1 | 0.5 | 0.5 | 0.25 | 1 |
| Methicillin susceptible | 2 | 0.5 | 0.5 | 0.25 | 0.125 | 1 |
| Methicillin susceptible | 2M* | 2 | 1 | 0.25 | 0.125 | 1 |
| Methicillin resistant | 3 | 1 | 0.5 | 0.25 | 0.25 | 1 |
| Teicoplanin intermediate | 4 | 1 | 1 | 0.5 | 0.25 | 16 |
| Teicoplanin intermediate | 4M | 4 | 2 | 1 | 0.25 | 16 |
| VISA | 5 | 4 | 2 | 1 | 0.25 | 16 |
| Staphylococcus epidermidis | ||||||
| Methicillin susceptible ATCC 12228 | 6 | 0.5 | 0.5 | 0.25 | 0.125 | 1 |
| Methicillin susceptible | 6M | 4 | 1 | 0.25 | 0.125 | 2 |
| Methicillin susceptible | 7 | 1 | 0.5 | 0.25 | 0.125 | 1 |
| Methicillin susceptible | 7M | 4 | 2 | 2 | 0.5 | 1 |
| Methicillin resistant | 8 | 1 | 0.5 | 0.25 | 0.125 | 2 |
| Methicillin resistant | 8M | 4 | 2 | 1 | 0.25 | 2 |
| Teicoplanin resistant | 9 | 1 | 1 | 0.25 | 0.125 | 8 |
| Teicoplanin resistant | 9M | 8 | 4 | 2 | 0.5 | 8 |
| Staphylococcus haemolyticus | ||||||
| Teicoplanin susceptible | 10 | 0.5 | 0.5 | 0.125 | 0.125 | 4 |
| Teicoplanin resistant | 11 | 0.5 | 0.5 | 0.25 | 0.125 | 16 |
| Teicoplanin resistant | 11M | 8 | 4 | 0.5 | 0.25 | 16 |
| Enterococcus faecalis ATCC 29212 | 12 | 4 | 2 | 1 | 0.125 | 0.25 |
| VanA 13 | 1 | 0.5 | 2 | 0.25 | 16 | |
| VanB 14 | 4 | 2 | 1 | 0.25 | 0.25 | |
| Enterococcus faecium ATCC 35667 | 15 | 2 | 1 | 2 | 0.25 | 0.5 |
| VanA 16 | 2 | 1 | 2 | 0.5 | >16 | |
| VanB 17 | 4 | 1 | 0.5 | 0.125 | 0.5 | |
| Listeria monocytogenes ATCC 19115 | 18 | 8 | 4 | 4 | 0.5 | 0.5 |
| 18M | >16 | 4 | 4 | 0.5 | 0.5 | |
| 19 | 8 | 2 | 2 | 0.25 | 0.5 | |
| Streptococcus pneumoniae | ||||||
| Penicillin susceptible ATCC 49619 | 20 | 0.125 | 0.125 | 0.125 | 0.03 | 0.125 |
| Penicillin susceptible | 20M | 1 | 1 | 0.125 | 0.06 | 0.125 |
| Penicillin susceptible | 21 | 1 | 0.5 | 0.125 | 0.03 | 0.125 |
| Penicillin susceptible | 21M | 4 | 0.5 | ND | ND | ND |
| Penicillin resistant | 22 | 0.5 | 0.25 | 0.125 | 0.03 | 0.125 |
| Streptococcus pyogenes ATCC 19615 | 23 | 1 | 0.5 | 1 | 0.125 | 0.125 |
| 24 | 1 | 0.5 | 0.06 | 0.03 | 0.125 | |
| Streptococcus agalactiae ATCC 13813 | 25 | 1 | 1 | 0.25 | 0.06 | 0.25 |
| 26 | 1 | 0.5 | 0.25 | 0.06 | 0.125 | |
M*, mutant strain with decreased susceptibility; VISA, vancomycin-intermediate Staphylococcus aureus; ND, not determined.
Comparisons of HMR 1043, daptomycin, and teicoplanin MICs by regression statistics yielded poor correlation coefficients as follows: HMR 1043 versus daptomycin, r = 0.51; HMR 1043 versus teicoplanin, r = 0.02. These data imply that HMR 1043 is more related to daptomycin than to teicoplanin.
Effect of calcium on activity.
The comparative MICs of HMR 1043 and daptomycin tested on MH agar and on CSMH are shown in Table 1. The addition of calcium had little effect on HMR 1043 activity: MICs generally varied no more than twofold. Daptomycin MICs determined in CSMH were fourfold lower than those obtained in unsupplemented MH agar: HMR 1043 versus daptomycin, r = 0.36.
Disk diffusion tests.
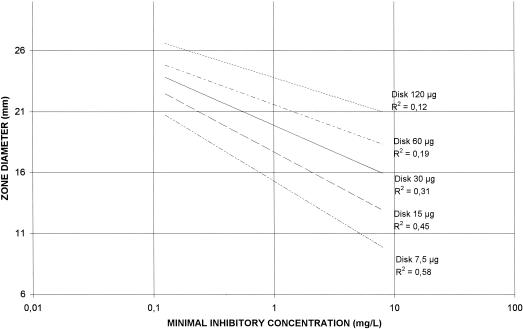
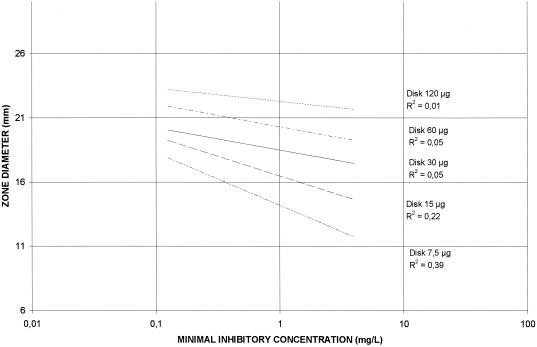
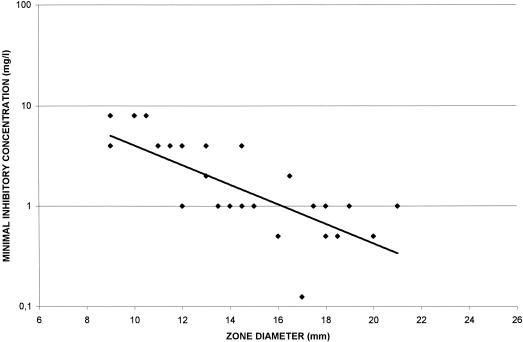
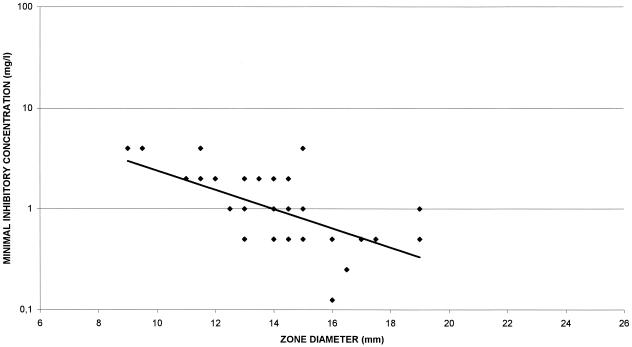
Correlations among HMR 1043 MICs and zone diameters around 7.5-,15-, 30-, 60-, and 120-μg HMR 1043 disks on MH agar and CSMH are shown in Fig. 2 and 3, respectively. The correlation coefficients for the four different disks were poor (r range, 0.12 to 0.58) and lower when disk diffusion tests were performed in CSMH (r range, 0.01 to 0.39). The best correlation was obtained with the 7.5-μg disk on MH agar (r = 0.58) versus 0.39 on CSMH. Scattergrams of the disk zone diameters and HMR 1043 MICs determined in MH agar and in CSMH are shown in Fig. 4 and 5, respectively. Therefore, zone diameters remained small: a MIC of 1 μg/ml was correlated with a zone diameter of 16 mm, and a MIC of 4 μg/ml was correlated with a zone diameter of 11 mm (Fig. 4). Disks with 7.5 μg of HMR 1043 performed better, with zone diameters very similar to those determined in MH agar.
FIG. 2.
Correlations between HMR 1043 MICs and zone diameters around 7.5-, 15-, 30-, 60-, and 120-μg HMR 1043 disks on MH agar.
FIG. 3.
Correlations between HMR 1043 MICs and zone diameters around 7.5-, 15-, 30-, 60-, and 120-μg HMR 1043 disks on CSMH.
FIG. 4.
Scattergram showing correlations between HMR 1043 MICs and zone diameters around 7.5-μg HMR 1043 disks on MH agar.
FIG. 5.
Scattergram showing correlations between HMR 1043 MICs and zone diameters around 7.5-μg HMR 1043 disks on CSMH.
HMR 1043 demonstrated good activity against susceptible and resistant gram-positive bacteria. The activity of HMR 1043 in vitro is less influenced by the presence of calcium ions than that of daptomycin. The activities of HMR 1043 and daptomycin against staphylococcal and streptococcal parental strains were similar, but daptomycin remains more active than HMR 1043 against enterococcal and listerial strains. Mutants selected from teicoplanin-intermediate or -resistant coagulase-negative staphylococci were 8-fold to 16-fold more resistant to HMR 1043 than their parental strains (MICs ≥ 4 μg/ml) and remained susceptible to daptomycin (MICs ≤ 2 μg/ml). Selection of in vitro susceptibility test breakpoints for HMR 1043 was difficult because of the poor correlation coefficients obtained with the different disks tested.
REFERENCES
- 1.Barry, A. L., P. C. Fuchs, and S. D. Brown. 2001. In vitro activities of daptomycin against 2,789 clinical isolates from 11 North American medical centers. Antimicrob. Agents Chemother. 45:1919-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coudron, P. E., J. L. Johnson, and G. L. Archer. 1987. In-vitro activity of LY146032 against Staphylococcus aureus and S. epidermidis. J. Antimicrob. Chemother. 20:505-511. [DOI] [PubMed] [Google Scholar]
- 3.Ehlert, F., and H. C. Neu. 1987. In vitro activity of LY146032 (daptomycin), a new peptolide. Eur. J. Clin. Microbiol. 6:84-90. [DOI] [PubMed] [Google Scholar]
- 4.Eliopoulos, G. M., S. Willey, E. Reiszner, P. G. Spitzer, G. Caputo, and R. C. Moellering. 1986. In vitro and in vivo activity of LY 146032, a new cyclic lipopeptide antibiotic. Antimicrob. Agents Chemother. 30:532-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs, P. C., A. L. Barry, and S. D. Brown. 2000. Daptomycin susceptibility tests: interpretive criteria, quality control, and effect of calcium on in vitro tests. Diagn. Microbiol. Infect. Dis. 38:51-58. [DOI] [PubMed] [Google Scholar]
- 6.Jones, R. N., and A. L. Barry. 1987. Antimicrobial activity and spectrum of LY146032, a lipopeptide antibiotic, including susceptibility testing recommendations. Antimicrob. Agents Chemother. 31:625-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louie, A., A. L. Baltch, W. J. Ritz, R. P. Smith, and M. Asperilla. 1993. Comparison of in vitro inhibitory and bactericidal activities of daptomycin (LY 146032) and four reference antibiotics, singly and in combination, against gentamicin-susceptible and high-level-gentamicin-resistant enterococci. Chemotherapy 39:302-310. [DOI] [PubMed] [Google Scholar]
- 8.Mobarakai, N., J. M. Quale, and D. Landman. 1994. Bactericidal activities of peptide antibiotics against multidrug-resistant Enterococcus faecium. Antimicrob. Agents Chemother. 38:385-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. 1997. Performance standards for antimicrobial susceptibility tests, 6th ed. Approved standard M2-A6. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 10.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 11.Rybak, M. J., E. Hershberger, T. Moldovan, and R. G. Grucz. 2000. In vitro activities of daptomycin, vancomycin, linezolid, and quinupristin-dalfopristin against staphylococci and enterococci, including vancomycin-intermediate and -resistant strains. Antimicrob. Agents Chemother. 44:1062-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snydman, D. R., N. V. Jacobus, L. A. McDermott, J. R. Lonks, and J. M. Boyce. 2000. Comparative in vitro activities of daptomycin and vancomycin against gram-positive pathogens. Antimicrob. Agents Chemother. 44:3447-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szybalski, W. 1952. Microbial selection. I. Gradient plate technique for study of bacterial resistance. Science 116:46-48. [Google Scholar]