Abstract
Pneumocystis jiroveci (human-derived P. carinii) is an opportunistic pathogenic fungus which causes pneumonia and is life-threatening in immunocompromised individuals. Spontaneously acquired resistance to atovaquone, a hydroxynaphthoquinone that is used to treat P. jiroveci infections, was linked to mutations in the mitochondrially encoded cytochrome b gene. Because P. jiroveci cannot be easily cultivated, we have developed Saccharomyces cerevisiae as an alternative system to study atovaquone resistance mutations. In this work, we introduced seven mutations linked with atovaquone resistance in P. jiroveci into the S. cerevisiae cytochrome b gene. The effects of the mutations on the respiratory function and on the sensitivity to the inhibitor were then characterized. Six of the reported mutations lowered the sensitivity of the S. cerevisiae bc1 complex to atovaquone, while one mutation had no effect on the drug resistance. These results were confirmed by monitoring the in vivo resistance of S. cerevisiae mutants which carried both the cytochrome b mutations and a deletion of the ABC transporter genes, allowing the drug to bypass the weakened efflux pump system. S. cerevisiae thus provides an easy-to-use system to characterize in vivo and in vitro cytochrome b mutations reported in pathogens and to assess their role in drug resistance.
The mitochondrial bc1 complex is a membrane-bound enzyme that catalyzes the transfer of electrons from ubiquinol to cytochrome c and couples this electron transfer to the vectorial translocation of proton across the inner mitochondrial membrane. The enzyme consists of 10 or 11 different polypeptides. Cytochrome b, cytochrome c1, and the Rieske iron-sulfur protein form the catalytic core of the enzyme. The catalytic mechanism, called the Q cycle, requires two distinct quinone-binding sites (Qo, quinol oxidation site, and Qi, quinone reduction site) which are located on opposite sides of the membrane and linked by a transmembrane electron transfer pathway. The mitochondrially encoded cytochrome b provides the quinol and quinone binding pockets and the transmembrane electron pathway.
A number of quinol antagonists that inhibit bc1 activity are known. They are specific for either the Qo site, such as myxothiazol, stigmatellin, and strobilurin, or the Qi site, such as antimycin, funiculosin, and diuron. Some bc1 inhibitors are widely used in agriculture and medicine as antiparasitic drugs. The hydroxynaphthoquinone atovaquone, for instance, is a broad-spectrum drug active against various parasitic infections, such as malaria, Pneumocystis jiroveci (human-derived P. carinii) pneumonia, and toxoplasmosis. Resistance to the drug was reported in Plasmodium spp. and Toxoplasma gondii isolated from patients who experienced a recrudescence of parasitemia after treatment with atovaquone, in parasites cultivated in vitro on selective medium, and in animals treated with the drug (8, 10, 13). Mutations in the cytochrome b gene of the pathogens were observed. These mutations were located in the Qo binding site pocket and correlated with an increased resistance to atovaquone.
Several mutations in the cytochrome b gene of P. jiroveci from AIDS patients have also been observed. P. jiroveci pneumonia is a common severe opportunistic infection in AIDS patients and a significant cause of mortality. A cohort study on AIDS patients with P. jiroveci infection suggested that exposure to atovaquone increased the frequency of cytochrome b mutations in the fungus (7). However, it is not possible to demonstrate a direct relationship between these cytochrome b mutations and the acquired resistance to atovaquone because P. jiroveci cannot be cultivated in medium or isolated in sufficient quantities for enzyme assay. Therefore, we developed Saccharomyces cerevisiae as a model organism to study the effects of the mutations observed in the fungus.
In this work, seven P. jiroveci mutations of conserved residues located in the Qo site of cytochrome b, T127I, I147V, T148I, L150F, S152A, P266L, and L275F (Fig. 1 and 2), were introduced into S. cerevisiae cytochrome b. The S. cerevisiae mutants were then used to assess the effect of these mutations on atovaquone resistance.
FIG. 1.
Sequence alignment of S. cerevisiae (Sc), P. jiroveci, and human-derived P. carinii (Pc) mitochondrial cytochrome b. Point mutations discussed in this paper are boxed in bold lines. Residues in close contact with stigmatellin in the S. cerevisiae bc1 complex crystal structure 1EZV (defining the Qo site) are indicated by a grey line above the sequence data. Residues corresponding to the tester mutations A144F and L263stop are circled.
FIG. 2.
Location of atovaquone resistance mutations in the bc1 structure. The structure was drawn with the coordinates of the S. cerevisiae enzyme (accession number PDB 1EZV) (6). The bound inhibitor stigmatellin is in green.
MATERIALS AND METHODS
Strains, media, and chemicals.
The following media were used for the growth of S. cerevisiae: YPD (1% yeast extract, 2% peptone, 3% glucose), YPG (1% yeast extract, 2% peptone, 3% glycerol), and transformation medium (0.7% yeast nitrogen base, 3% glucose, 2% agar, 1 M sorbitol and 0.8 g of a complete supplement mixture minus uracil per liter, supplied by Anachem).
The following S. cerevisiae strains were used for transformation and screening: the recipient strain for biolistic transformation was W303-1B/rho0 (MATα ura3 his3 leu2 ade2 trp1); the mit− tester strains CKB78 (CYTB A144F) and CKL57 (CYTB L263Stop) were MATa leu1 kar1-1 with a rho+ intronless mitochondrial DNA. JC8/56 (MATa leu1 kar1-1 rho0) was used for cytoduction. Strains carrying single deletions of YOR1, SNQ2, PDR5, PDR10, PDR11, YCF1, PDR3, PDR15, and PDR1 were obtained from Euroscarf (http://www.uni-frankfurt.de/fb15/mikro/euroscarf/). The strain AD1-9, harboring multiple deletions (MATa ura3 his1 yor1Δ::hisG snq2Δ::hisG pdr5Δ::hisG pdr10Δ::hisG pdr11Δ::hisG ycf1Δ::hisG pdr3Δ::hisG pdr15Δ::hisG pdr1Δ::hisG) (4) was kindly given by M. Ghislain (UCL, Belgium). These strains were used to monitor atovaquone sensitivity in vivo. AD1-9/rho0 was obtained by ethidium bromide treatment.
2,3-Dimethoxy-5-methyl-n-decyl-1,4-benzoquinol and myxothiazol were purchased from Sigma. Stigmatellin was purchased from Fluka. Atovaquone was obtained from Glaxo-Wellcome.
Site-directed mutagenesis, biolistic transformation, and selection of mitochondrial mutants.
Plasmid pBM5 carrying the wild-type intronless sequence of the CYTB gene was constructed by blunt-end cloning of a PCR product of CYTB into the pCRscript vector (Stratagene). The mutagenesis was performed with the Quickchange site-directed mutagenesis kit (Stratagene) according to the manufacturer's recommendations. After verification of the sequence, the plasmids carrying the mutated genes were used for biolistic transformation.
Mitochondrial transformation by microprojectile bombardment was adapted from reference 1. Approximately 3 μg of the plasmids carrying the mutated CYTB genes and 0.5 μg of plasmid YEP352 (which contains the URA3 gene, allowing the selection of Ura+ nuclear transformants) were mixed with 50 μl of 0.7-μm tungsten particles (at a concentration of 60 mg/ml). The particles (supplied by Bio-Rad) were prepared and coated with DNA according to the manufacturer's protocol. Aliquots of the coated particles were used for transformation of the recipient strain W303-1B/rho0. Around 107 cells of the recipient strain were evenly spread on transformation medium (selective for Ura+ transformants). The particle bombardments were performed with a Biolistic PDS-1000/He particle delivery system (Bio-Rad) according to the manufacturer's recommendations.
Colonies of Ura+ transformants (which have received plasmid YEP352) appeared after 4 to 5 days of incubation. The Ura+ colonies were crossed with respiratory-deficient (mit−) tester strains CKB78(A144F) and CKL57(L263Stop), which carry mutations located near the atovaquone resistance mutations (Fig. 1). CKB78(A144F) was used for mutations in residues 127 to 152, whereas CKL57(L263Stop) was used for mutations in residues 266 and 275. The diploids were replica-plated on respiratory medium (YPG). Since the tester deficiency mutations could be corrected by recombination with the plasmid-derived mitochondrial sequence, mitochondrial transformants were identified by their ability to form respiration-competent diploids when crossed with the tester strains. Respiration-competent diploids that issued from the crossing were selected and subcloned. Their sequencing showed that the new mutations were incorporated while the tester mutations were replaced by the wild-type codon.
Construction of S. cerevisiae strains with combined atovaquone resistance mutations and deletion of membrane transporters.
The mitochondrial genome carrying the atovaquone resistance mutations was transferred into strain AD1-9 as follows. The mitochondrial transformants which carried the atovaquone resistance mutations were sporulated. The haploid clones were used for cytoduction of the mitochondrial genome into the recipient strain JC8/56 (rho0). This strain carries the mutation kar1-1, which is required for cytoduction (3). These cytoductants were then used to transfer the mitochondrial genome into AD1-9/rho0. The resulting strains combined atovaquone mutations and multiple deletions in the membrane transporters and were used to monitor atovaquone resistance in vivo.
Preparation of mitochondrial membrane, cytochrome c reductase activity, and inhibitor titrations.
Mitochondrial membranes were isolated as described before (12). Cytochrome c reductase activity measurements were assayed in 50 mM potassium phosphate (pH 7.0), 250 mM sucrose, 0.2 mM EDTA, 1 mM NaN3, 2.5 mM KCN, 0.01% Tween 20, and 40 μM cytochrome c (assay buffer) at 23°C. Membranes were diluted to 2.5 nM cytochrome bc1 complex. The reaction was started by adding 50 μM 2,3-dimethoxy-5-methyl-n-decyl-1,4-benzoquinol, an analogue of ubiquinol. Reduction of cytochrome c was monitored in an Aminco DW-2a spectrophotometer at 550 versus 539 nm in dual-wavelength mode. Data were collected and analyzed with an Online Instrument Systems Inc. computer interface and software. Activities of the bc1 complex in membranes were measured in duplicate, and turnover numbers were calculated on the basis of cytochrome b concentration. The cytochrome b content was estimated from reduced minus oxidized spectra, with the extinction coefficient 25 mM−1 cm−1 at 562 to 572 nm.
Spectroscopic analysis of cytochromes in whole cells.
Spectra were generated by scanning cell suspensions with a single-beam spectrophotometer built in-house. The cells, grown on YPG plates for 48 h, were resuspended in 5% Ficoll at a concentration of around 200 mg of cells per ml and reduced with dithionite. A quadratic baseline compensation was carried out on the data as described before (2) to remove the distortion of the baseline.
RESULTS AND DISCUSSION
Generation of S. cerevisiae mutants harboring cytochrome b mutations observed in P. jiroveci.
Several mutations in the cytochrome b gene of P. jiroveci were reported following exposure to atovaquone. The mutations T127I, I147V, T148I, L150F, S152A, P266L, and L275F (S. cerevisiae numbering) are located in the Qo binding site (Fig. 1 and 2). Because P. jiroveci cannot be cultivated in medium, a model organism is needed to assess the role of the mutations in atovaquone resistance. S. cerevisiae is the obvious choice. S. cerevisiae is amenable to mitochondrial transformation, so that designed mutations can be introduced in the mitochondrially encoded cytochrome b. In addition, the sequences of cytochrome b from S. cerevisiae and P. jiroveci are highly similar (63% identity), as shown in Fig. 1. The P. jiroveci mutations were therefore introduced into S. cerevisiae by the biolistic technique as described in Materials and Methods. The effects of the mutations on respiratory competence and resistance to inhibitor were then monitored.
Effect of mutations on respiratory competence.
The growth rate of the wild-type and mutant strains in respiratory medium (YPG) was monitored. The mutations had no effect on the respiratory competence of the cells, since the doubling times on a nonfermentable carbon source of the control and mutant strains were identical (≈5 h). Similarly, no significant decrease in oxygen consumption was observed in the mutant cells (data not shown). Analysis of the optical signal of the cytochromes in whole cells showed a slight decrease in the cytochrome b content in the mutants compared with the wild-type strain. The cytochrome b level was decreased by approximately 15% in all the mutants except T148I, where the cytochrome b content was decreased by approximately 30%. Figure 3 shows the optical spectra of the wild-type strain and two mutants: T148I, which had a low cytochrome b content, and I147V. The optical spectra of the remaining mutants were almost identical to that of I147V. Thus, it seems that the introduction of the mutations slightly affect the folding of cytochrome b and/or the assembly of acomplex.
FIG. 3.
Optical spectra of wild-type, L148I, and L147V cells. Optical spectra of reduced cell suspensions were obtained as described in Materials and Methods. c, cytochrome c (heme c); b, cytochrome bc1 (hemes b); a, cytochrome oxidase (hemes aa3).
Mitochondrial membranes were prepared from the wild-type and mutant strains, and the ubiquinol-cytochrome c reductase activity of the bc1 complex in the membranes was monitored as described in Materials and Methods (Table 1). The activity in the absence of inhibitor was 48 s−1 for the wild-type enzyme. The introduction of the T127I mutation had little effect, since the activity was 81% of that of the wild type. In I147V, T148I, L150F, P266L, and L275F, the activity was decreased by 33 to 55%. The mutation S152A seemed to have a more deleterious effect on the activity, since it was decreased to 20% of that of the wild type. The decrease in the bc1 activity was not unexpected, since the mutations affected residues in the Qo site of the enzyme, which is essential for its catalytic activity.
TABLE 1.
Cytochrome c reductase activities of mitochondrial membranes and sensitivity to atovaquone
| Strain | Activity (% of wild type)a | Atovaquone I50 (nM)b |
|---|---|---|
| Wild type | 100 | 50 |
| T127I | 81 | 50 |
| I147V | 67 | 100 |
| T148I | 58 | 100 |
| L150F | 54 | 150 |
| P266L | 45 | 350 |
| L275F | 52 | 400 |
| S152A | 20 | >400 |
The cytochrome c reductase activities were measured as described in Materials and Methods.
The I50 is the concentration of atovaquone required to obtain 50% inhibition of the activity.
T127, I147, T148, and L150 are very highly conserved residues in mitochondrial cytochrome b. P266 and L275 are completely conserved in the available mitochondrial sequence data. S152 is less well conserved in eukaryotes and is replaced by another hydroxylated residue, such as tyrosine or threonine, in some species. The S152A mutation, however, had the more deleterious effect. The decreased activity might be explained by the lost of the hydroxyl group of residue 152, which could hydrogen bond with the amide side chain of N149 and stabilize the structure. The introduction of the bulky phenyl side chain in the L150F mutant might be expected a priori to be especially detrimental. However, the structure seems to be able to accommodate a large residue in that region, since the bc1 activity of the mutant was still 54% of the wild-type activity.
The decrease in both cytochrome b content and bc1 activity in the mutant cells was not enough to affect the respiratory growth of the mutants. The introduction of these mutations had little or no effect on the overall fitness of these strains. It is likely that the mutations do not have severely deleterious effects in P. jiroveci, because a major decrease in respiratory competence would be lethal for the fungus.
Effect of mutations on sensitivity of bc1 complex to atovaquone.
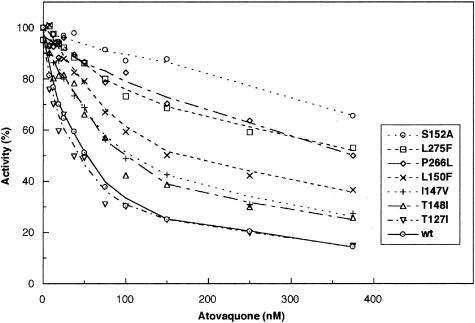
In order to assess the role of the mutations on atovaquone resistance, mitochondrial membranes were prepared from the wild-type and mutant strains, as described above, and the cytochrome c reductase activity was monitored in the presence of increasing concentrations of atovaquone (Fig. 4). Atovaquone is an efficient inhibitor of the S. cerevisiae bc1 complex because the inhibitor concentration required for 50% inhibition of the activity (I50) was around 50 nM. This was in agreement with data obtained with other organisms and with other methods: the concentration required for a 50% decrease in the membrane potential in Plasmodium yoelii was 15 nM (13), and a 50% decrease in nucleic acid synthesis activity was observed in the presence of 20 and 50 nM atovaquone in Plasmodium berghei and Toxoplasma gondii, respectively (14, 10).
FIG. 4.
Effect of atovaquone on bc1 activity of wild-type (wt) parental and mutant strains. The cytochrome c reductase activity of mitochondrial membranes was measured as described in Materials and Methods in the presence of increasing concentrations of inhibitor. The assays were performed in duplicate, and the results are shown as averages, with less then 10% variation in individual rates. Activities are expressed as a percentage of the turnover number of each strain in the absence of atovaquone.
It is likely that the atovaquone binds in the same domain of the Qo pocket as stigmatellin. The Qo site of cytochrome b is a rather large domain formed from components encompassing amino acid residues 120 to 150 and 260 to 280 of the polypeptide chain. It consists of two lobes, a heme bl proximal lobe and a distal lobe. The distal lobe is close to the surface region of cytochrome b involved in interactions with the peripheral domain of the iron-sulfur protein. The stigmatellin head group binds in this distal domain and is positioned in a pocket formed by the regions from 122 to 131, 142 to 152, and 268 to 280 (Fig. 1). All interactions with the iron-sulfur protein occur in this region. The pharmacophore side chains of myxothiazol and monoamine oxidase-type inhibitors, by contrast, occupy the proximal domain. Since atovaquone affects the midpoint potential and electron paramagnetic resonance spectrum of the iron-sulfur protein (T. Merbitz-Zahradnik and B. Trumpower, unpublished observations), it is likely that atovaquone binds in the distal domain, similar to stigmatellin.
It is interesting that the P. jiroveci mutations studied here are located in or close to the inhibitor binding regions (Fig. 1). It was therefore expected that they might modify the sensitivity of the bc1 complex to atovaquone. In addition, mutations of residues L275 and I147, conferring resistance to other Qo inhibitors in S. cerevisiae, were reported previously (5). As shown in Fig. 4, three mutants, T148I, I147V, and L150F, showed a slight decrease in sensitivity to atovaquone. The I50 increased to approximately 100 to 150 nM. Three mutants, P266L, L275F, and S152A, showed a marked resistance, with I50s higher than 350 nM. Surprisingly, T127I did not show any increased resistance to atovaquone.
The threefold increase in resistance observed with the L150F mutant is undoubtedly due to steric hindrance arising from the bulky aromatic side chain of the introduced phenyl group. Similarly, P266L and L275F, which confer high atovaquone resistance, result in the introduction of bulkier nonpolar side chains, which may sterically hinder antagonist binding. I147V represents a conservative structural change. The loss of a side chain methylene moiety may remove a potentially stabilizing hydrophobic interaction with atovaquone bound at the Qo site or may subtly affect the packing of the protein around this region, which forms a tunnel for access to the lipid phase. The mutations S152A and T148I both result in replacement of a residue with a polar, hydroxylated side chain by a nonpolar, aliphatic species, although the resistance of S152A is much higher than that of T148I. It is possible that the mutations abolish hydrogen bond interactions involving S152 or T148 and result in slight structural rearrangement of components within the Qo site. Molecular modeling studies with minimum energy calculations to assess structural changes in the protein resulting from the resistance mutations are currently in progress.
Because of the high similarity between S. cerevisiae and P. jiroveci cytochrome b, it is expected that the mutations have similar effects on the pathogen's bc1 complex. The mutation T127I probably has little effect on the bc1 sensitivity to atovaquone in P. jiroveci. As shown above, this mutation also had little effect on the activity of the complex. It is tempting to suggest that the change is a silent mutation. The mutations I147V, T148I, and L150F would confer a slight advantage to the mutant fungi when exposed to atovaquone, whereas the mutations P266L, L275F, and S152A would cause a marked resistance to treatment with the drug.
Sensitivity of S. cerevisiae cells to atovaquone treatment.
As shown in Fig. 4, the molecular target of atovaquone, the bc1 complex, is inhibited by submicromolar concentrations of atovaquone. However, S. cerevisiae is naturally resistant to the drug. At 50 μM atovaquone, no decrease in respiratory growth was observed for the control strain (Fig. 5). Since it was likely that the resistance of the S. cerevisiae cells to atovaquone treatment was due to efficient efflux pumps, we monitored the sensitivity of strains harboring deletions of genes involved in drug resistance, namely YOR1, SNQ2, PDR5, PDR10, PDR11, YCF1, PDR3, PDR15, and PDR1. These genes are members of gene families which, in mammalian cells, are involved in the resistance of tumor cells to anticancer treatment. Similar mechanisms exist in pathogenic fungi, such as Candida and Aspergillus spp. (11, 15). PDR1 and PDR3 code for transcriptional regulators. Yor1p, Snq2p, Pdr5p, Pdr10p, Pdr11p, Ycf1p, and Pdr15p are ABC transporters (reviewed in reference 16). These plasma membrane transporters, especially Pdr5p and Snq2p, mediate the ATP-dependent efflux of a large number of unrelated compounds.
FIG. 5.
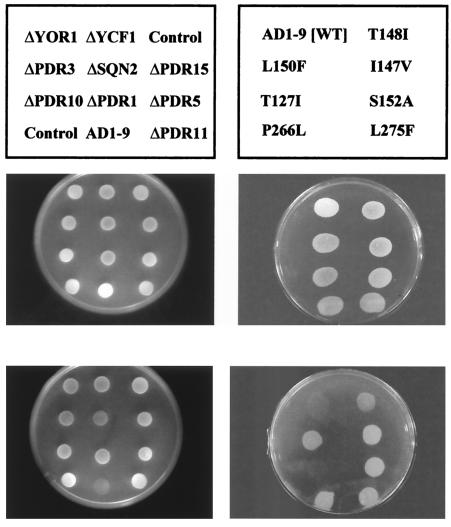
Sensitivity of S. cerevisiae cells to atovaquone exposure. The name and position of the strains tested are shown above the plates. ΔYOR1, ΔYCF1, ΔPDR3, ΔSNQ2, ΔPDR15, ΔPDR10, ΔPDR1, ΔPDR5, and ΔPDR11 are strains carrying single deletions of genes of the ABC transporter family; AD1-9 carries deletions of all these genes (left-hand panels). The right-hand panels show the growth of AD1-9 and the seven strains derived from AD1-9 which carry cytochrome b mutations. WT, wild type.
As shown in Fig. 5, none of the single deletions resulted in a dramatic increase in the sensitivity to atovaquone. Only a slight decrease in respiratory growth was observed. By contrast, the deletion of the nine genes in strain AD1-9 (Δyor1, Δsnq2, Δpdr5, Δpdr10, Δpdr11, Δycf1, Δpdr3, Δpdr15, and Δpdr1) severely increased the sensitivity of the cells to the drug. It is therefore likely that atovaquone can be pumped out across the plasma membrane by two or more transporters and that a clear phenotype is observed when all the transporters involved in its efflux are inactivated. This was observed for other compounds. Pdr5p and Snq2p, for example, have overlapping transport activity for steroids (9). Yor1p and Pdr5p also share several substrates (4). The identification of the exact mechanism causing resistance in S. cerevisiae is beyond the scope of this study but would be interesting because the equivalent genes in P. jiroveci and other pathogens could be involved in resistance to atovaquone treatment.
The multiple deletion of genes involved in multidrug resistance resulted in an increased sensitivity to atovaquone. The AD1-9 strain, which combines the inactivation of the ABC transporters and a wild-type cytochrome b, was indeed unable to grow in the presence of atovaquone. This hypersensitive strain could then be used to monitor in vivo the drug resistance caused by cytochrome b mutations. For this purpose, the seven mutant strains and a parental (wild-type) strain were constructed as described in Materials and Methods. They derived from AD1-9 but harbored the P. jiroveci cytochrome b mutations mentioned above. As shown in Fig. 5, T127I did not confer any resistance to the drug, whereas the other mutations resulted in clear resistance to the inhibitor. These data are in agreement with the results obtained in our in vitro studies. Such S. cerevisiae strains could be useful tools to screen new compounds and to test the cross-resistance of existing mutants.
In conclusion, we have shown here that S. cerevisiae provides an easy-to-use system to characterize cytochrome b mutations observed in pathogens and to assess their role in drug resistance. Since S. cerevisiae is amenable to mitochondrial transformation, mutations found in cytochrome b in pathogens can be introduced into the S. cerevisiae mitochondrial gene. In addition, the availability of strains with multiple deletions of membrane transporters allows the in vivo study of drug resistance. The characterization of the resistance mutations in the pathogens and in model organisms such as S. cerevisiae could provide molecular markers to monitor the potential resistance of pathogens and screening tools for new inhibitors.
Acknowledgments
This work was supported by a Medical Research Fellowship and a BBSRC grant to B.M. and by National Institutes of Health grants AI46966 to S.M. and GM20379 to B.L.T. P.H. has a BBSRC Studentship.
REFERENCES
- 1.Bonnefoy, N., and T. D. Fox. 2001. Genetic transformation of Saccharomyces cerevisiae mitochondria. Methods Cell. Biol. 65:381-396. [DOI] [PubMed] [Google Scholar]
- 2.Brown, S., A. M. Colson, B. Meunier, and P. R. Rich. 1993. Rapid screening of cytochromes of respiratory mutants of Saccharomyces cerevisiae—application to the selection of strains containing novel forms of cytochrome c oxidase. Eur. J. Biochem. 213:137-145. [DOI] [PubMed] [Google Scholar]
- 3.Conde, J., and G. R. Fink. 1976. A mutant of Saccharomyces cerevisae defective for nuclear fusion. Proc. Natl. Acad. Sci. USA 73:3651-3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Decottignies, A., A. M. Grant, J. W. Nichols, de Wet, H., D. B. McIntosh, and A. Goffeau. 1998. ATPAse and multidrug transporter activities of the overexpressed yeast ABC protein Yor1p. J. Biol. Chem. 273:12612-12622. [DOI] [PubMed] [Google Scholar]
- 5.diRago, J. P., J. Y. Coppé, and A. M. Colson. 1989. Molecular basis for resistance to myxothiazol, mucidin (strobilurin A) and stigmatellin. J. Biol. Chem. 264:14543-14548. [PubMed] [Google Scholar]
- 6.Hunte, C., J. Koepke, C. Lange, T. Rossmanith, and H. Michel. 2000. Structure at 2.3 angstrom resolution of the cytochrome bc1 complex from the yeast Saccharomyces cerevisiae cocrystallized with an antibody Fv fragment. Structure Fold. Des. 8:669-684. [DOI] [PubMed] [Google Scholar]
- 7.Kazanjian, P., W. Armstrong, P. A. Hossler, L. Huang, C. B. Beard, J. Carter, L. Crane, J. Duchin, W. Burman, J. Richardson, and S. R. Meshnick. 2001. Pneumocystis carinii cytochrome b mutations are associated with atovaquone exposure in patients with AIDS. J. Infect. Dis. 183:819-822. [DOI] [PubMed] [Google Scholar]
- 8.Korsinczky, M., N. Chen, B. Kotecka, A. Saul, K. Rieckmann, and Q. Cheng. 2000. Mutations in Plasmodium falciparum cytochrome b that are associated with atovaquone resistance are located at a putative drung-binding site. Antimicrob. Agents Chemother. 44:2100-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahe, Y., Y. Lemoine, and K. Kuchler. 1996. The ATP binding cassette transporters Pdr5 and Snq2 of Saccharomyces cerevisae can meditate transport of steroids in vivo. J. Biol. Chem. 271:25167-25172. [DOI] [PubMed] [Google Scholar]
- 10.McFadden, D. C., S. Tomavo, E. A. Berry, and J. C. Boothroyd. 2000. Characterization of cytochrome b from Toxoplasma gondii and Qo domain mutations as a mechanism of atovaquone-resistance. Mol. Biochem. Parasitol. 108:1-12. [DOI] [PubMed] [Google Scholar]
- 11.Sanglard, D., F. Ischer, and J. Bille. 2001. Role of ATP-Binding-Cassette transporter genes in high-frequency acquisition of resistance to azole antifungals in Candida glabrata. Antimicrob. Agents Chemother. 45:1174-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitt, M. E., and B. L. Trumpower. 1991. The petite phenotype resulting from a truncated copy of subunit 6 results from loss of assembly of the cytochrome bc1 complex and can be suppressed by overexpression of subunit 9. J. Biol. Chem. 266:14958-14963. [PubMed] [Google Scholar]
- 13.Srivastava, I. K., J. M. Morrisey, E. Darrouzet, F. Daldal, and A. B. Vaidya. 1999. Resistance mutations reveal the atovaquone-binding domain of cytochrome b in malaria parasites. Mol. Microbiol. 33:704-711. [DOI] [PubMed] [Google Scholar]
- 14.Syafruddin, D., J. E. Siregar, and S. Marzuki. 1999. Mutations in cytochrome b gene of Plasmodium berghei conferring resistance to atovaquone. Mol. Biochem. Parasitol. 104:185-195. [DOI] [PubMed] [Google Scholar]
- 15.Vanden Bossche, H., F. Dromer, I. Improvisi, Lozano-Chiu, M., J. H Rex, and D. Sanglard. 1998. D. Antifungal drug resistance in pathogenic fungi. Med. Mycol. 36:116-128. [PubMed] [Google Scholar]
- 16.Wolfger, H., Y. Mamnun, and K. Kuchler. 2001. Fungal ABC proteins: pleitropic drug resistance, stress response and cellular detoxification. Res. Microbiol. 152:375-389. [DOI] [PubMed] [Google Scholar]