Abstract
When Pseudomonas aeruginosa PAO1 is treated with gentamicin, it releases membrane vesicles containing gentamicin (g-MVs) and peptidoglycan hydrolase, which makes the MVs bactericidal. We evaluate the ability of g-MVs to deliver gentamicin past the intrinsic permeability barrier of group IIIa Burkholderia cepacia and show that strain CEP0248 with low resistance to gentamicin is killed but the highly resistant strain C5424 is not. Immunoelectron microscopy revealed that gentamicin was delivered into both strains, suggesting that there might be another mechanism of resistance in C5424.
Together with Pseudomonas aeruginosa, Burkholderia cepacia is a primary opportunistic pathogen of cystic fibrosis patients. Infection with B. cepacia is generally associated with aggressive necrotizing pneumonia and is accompanied by an acute systemic infection, such as bacteremia or septicemia (8). This rapid clinical decline due to B. cepacia colonization is known as the so-called “cepacia syndrome” and leads to mortality in 20 to 35% of chronically infected individuals (9). Treatment is often made more difficult due to the innate impermeability of B. cepacia's outer membrane (OM) to antibiotics like aminoglycosides, polymyxin, and β-lactams (7, 17, 25).
One potential method of circumventing this resistance is through the use of a specific membrane-based antibiotic delivery system, such as gentamicin-containing membrane vesicles (g-MVs) that can breach the OM. The g-MVs from P. aeruginosa PAO1 successfully deliver gentamicin and a peptidoglycan hydrolase into both gram-positive and -negative bacteria (11, 12, 16). For gram-negative pathogens, g-MVs contact the bacterium's OM and fuse into it so as to release the vesicle's contents into the periplasm of the cell. Here, the (now) periplasmic gentamicin is actively taken into the cytoplasm to inhibit protein synthesis. At the same time, the (now) periplasmic peptidoglycan hydrolase begins to hydrolyze the host's peptidoglycan layer. This two-pronged attack by the g-MVs might be an attractive system to use against pathogens that are intrinsically impermeable to antibiotics, especially since g-MVs are thermodynamically stable and do not break down in suspension (12, 16). The aim of the present work was to study the utility of g-MVs against B. cepacia. Strains C5424 and CEP0248 were chosen as the test strains because they represent members of the B. cepacia group IIIa complex, which comprises 80% of the B. cepacia clinical isolates in Canada (22). Additionally, these two distinct strains possess smooth lipopolysaccharide (LPS) and cable (Cbl) pili, suggesting that they might present similar surfaces for g-MV attachment. The striking difference between the strains is in their susceptibility to gentamicin. The MIC of gentamicin for CEP0248 is 5 μg/ml, while that for C5424 is 20 μg/ml.
The g-MVs were generated from P. aeruginosa PAO1 as described previously (12) and contained 7.0 (±1.0) ng of gentamicin/μg of MV protein as estimated by enzyme-linked immunosorbent assay (12, 16). The killing potential of these g-MVs was monitored by performing viable plate counts after treatment (11, 13, 16). No growth was taken as cell death due to the action of soluble gentamicin or g-MVs.
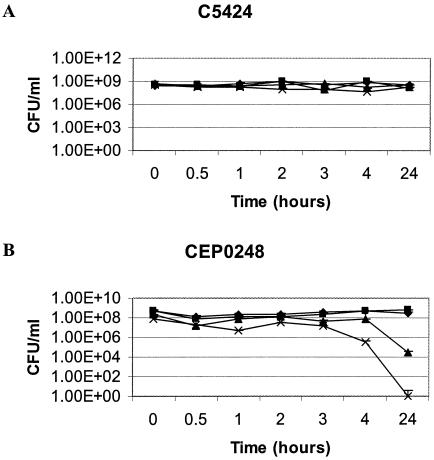
From the viability studies (Fig. 1), it is clear that C5424 was not susceptible to g-MVs, naturally produced MVs not containing gentamicin (n-MVs), or soluble gentamicin at a dose of 2.5 times the MIC over the test period. CEP0248 showed a viability decline with soluble gentamicin at 4 h and complete viability loss at 24 h. A similar trend was also noted with g-MVs, with a 4 to 5 log10-fold decrease in viability at 24 h. Both strains showed no significant viability loss over 24 h under control or n-MV conditions.
FIG. 1.
Bactericidal assays to monitor the effects on B. cepacia C5424 (A) and CEP0248 (B) of soluble gentamicin at 2.5 times the MIC (×), PAO1 n-MVs (▪), PAO1 g-MVs (▴), and a HEPES buffer control (⧫). The MVs were added at a protein concentration of 100 μg/ml, and error bars represent standard deviations.
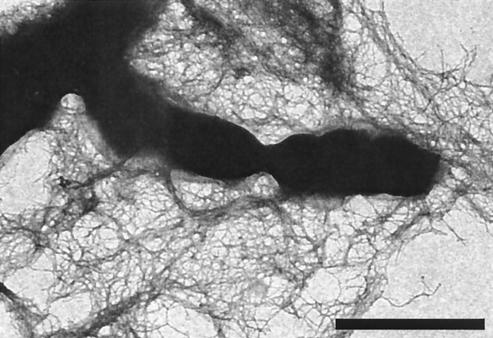
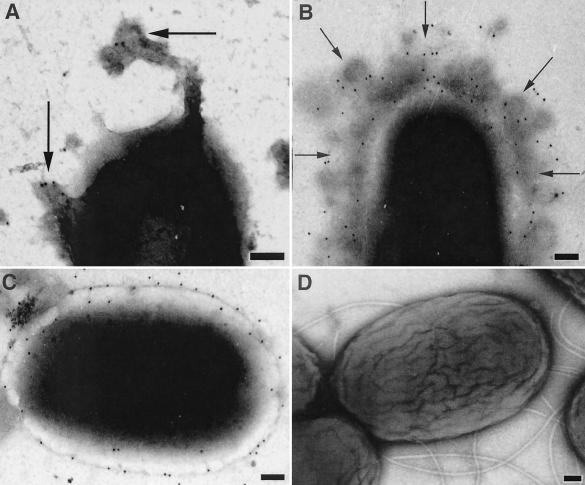
One of the potential barriers to effective cellular binding of g-MVs might come from the extensive network of Cbl pili (20) produced by B. cepacia (Fig. 2). In order to assess the binding of the g-MVs to B. cepacia, transmission electron microscopy (TEM) was employed to view samples taken at specific time points in the bactericidal assay (Fig. 3). B. cepacia produces its own n-MVs (N. Allan and T. Beveridge, submitted for publication), and this, of course, complicates the monitoring of exogenous g-MVs from PAO1. By taking advantage of the fact that the g-MVs from PAO1 possess immunogenically distinct B-band LPS on their surfaces (11-13, 15), we employed gold-conjugated anti-B-band monoclonal antibodies (anti-B MAbs) (12) to distinguish between PAO1 g-MVs and B. cepacia n-MVs. In this way, PAO1 g-MVs could be monitored by TEM throughout the bactericidal assay. Since C5424 retained its resistance to gentamicin whereas CEP0248 did not, we show only the results for C5424 (i.e., we wanted to ensure that the PAO1 g-MVs were interacting with C5424 cells). No cross-reactivity to B. cepacia LPS was observed with the anti-B MAbs (Fig. 3D). After B-band LPS was detected, the binding and fusion of PAO1 g-MVs to C5424 (Fig. 3A and B) and their subsequent integration into the OM (Fig. 3C) were seen.
FIG. 2.
Negatively stained whole mount of C5424 showing the delicate Cbl pili that are removed by washing during the immunogold labeling described in the legend to Fig. 3. Scale bar, 1 μm.
FIG. 3.
TEM images of negatively stained C5424 cells labeled with gold-conjugated anti-B MAbs towards PAO1 LPS. (A) Image from the antibactericidal assay at time zero. Arrows point to labeled MVs in the process of attaching to a cell. (B) Image taken at 1 h. Arrows show labeled MVs. (C) Image taken at 24 h. The gold label can be seen throughout the surface of this cell, indicating that the PAO1 g-MVs have fused the membrane and the PAO1 LPS has spread over the cell surface. (D) Control cells labeled with anti-B MAbs but without PAO1 g-MVs added. Scale bars, 100 nm.
In order to monitor the delivery of gentamicin via g-MVs, we prepared thin sections from samples taken at different time points as described previously (16). Gold-conjugated antibodies to gentamicin (see Sigma data sheet on product G 1015 for details of specific antibody activity) were used to detect the antibiotic within the g-MVs and to monitor the antibiotic's dissemination throughout the cells (Fig. 4B and C). Negative controls using PAO1 n-MVs revealed no cross-reactivity with the antigentamicin (Fig. 4A). Since g-MVs were able to successfully deliver gentamicin into the cytoplasm of intact C5424 cells and yet no reduction in the number of CFU was seen in the bactericidal assay, we believe that there must be alternative resistance mechanisms at work in addition to impermeability. Such mechanisms might include enzymatic degradation of the drug via plasmid-encoded enzymes (1), active efflux pumps (24), or altered ribosomes (6). At present we have no information about alternative resistance mechanisms in C5424.
FIG. 4.
Thin sections of C5424 labeled with gold-conjugated antibodies to gentamicin. (A) Control treated with n-MVs from PAO1; (B and C) g-MVs fusing to the OM. Small arrows show MVs containing gentamicin, whereas the large arrow in panel C shows gentamicin within the cytoplasm. Scale bars, 100 nm.
This study demonstrates the successful use of P. aeruginosa g-MVs in producing a 4 to 5 log10-fold decrease in viability at 24 h in CEP0248, which has an intermediate resistance to gentamicin. While the use of soluble gentamicin resulted in a complete loss in viability, the g-MV delivery system produced a marked decrease in viability with a mere fraction (7 ng/μg of MV protein) of the usual dose. Another favorable advantage to using g-MVs as a delivery vehicle was described in a previous study (10) where g-MVs from Shigella flexneri were used to successfully deliver gentamicin to intracellular S. flexneri organisms infecting a Henle tissue cell line. Evidence suggests that B. cepacia might be an opportunistic intracellular pathogen (2, 19), a possibility that highlights the potential benefits of using g-MVs to treat intracellular B. cepacia infections.
Our study also demonstrates the successful fusion of MVs from P. aeruginosa to B. cepacia. These organisms have been shown to form mixed biofilms when they coinfect the lungs of cystic fibrosis patients (19, 23). It is conceivable that, under the conditions of a mixed microbial community, MVs are constantly being shed by both organisms, allowing for the exchange of plasmids (5, 26) and periplasmic inactivating enzymes (3), and may even participate in quorum sensing by facilitating the exchange of large 12-C acylhomoserine-lactones (4, 14, 18, 21).
Acknowledgments
We thank Bob Harris, Dianne Moyles, and Anu Saxena of our laboratory for their excellent technical help with assays and TEM and Chris Whitfield, Anthony Clarke, and Kelly MacDonald of our department for advice. Additionally, we thank David Speert (Department of Paediatrics, University of British Columbia, Vancouver, Bristish Columbia, Canada) for B. cepacia strains.
This research was funded through a CBDN-NCE grant to T.J.B. The TEM was performed in the NSERC Guelph Regional STEM Facility, which is located in the Department of Microbiology and which is partially funded through an NSERC Major Facilities Access grant to T.J.B.
REFERENCES
- 1.Bresesinska, M., R. Benveniste, J. Davies, P. J. L. Daniels, and J. Weinstein. 1972. Gentamicin resistance in strains of Pseudomonas aeruginosa mediated by enzymatic N-acetylation of the deoxystreptoamine moiety. Biochemistry 11:761-765. [DOI] [PubMed] [Google Scholar]
- 2.Burns, J. L., J. Mechthild, E. Y. Chi, D. K. Clark, A. Berger, and A. Griffith. 1996. Invasion of respiratory epithelial cells by Burkholderia (Pseudomonas) cepacia. Infect. Immun. 64:4054-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coifu, O., T. J. Beveridge, J. Kadurugamuwa, J. Walther-Rosmussen, and N. Høiby. 2000. Chromosomal β-lactamase is packaged into membrane vesicles from Pseudomonas aeruginosa. J. Antimicrob. Chemother. 45:9-13. [DOI] [PubMed] [Google Scholar]
- 4.Conway, B.-A. D., V. Venu, and D. P. Speert. 2002. Biofilm formation and acyl homoserine lactone production in the Burkholderia cepacia complex. J. Bacteriol. 184:5678-5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorward, D. W., C. F. Garon, and R. C. Judd. 1989. Export and intercellular transfer of DNA via membrane blebs of Neisseria gonorrhoeae. J. Bacteriol. 171:2499-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garrod, L. P., H. P. Lambert, and F. O'Grady. 1981. Antibiotics and chemotherapy, 5th ed., p. 115-154. Churchill Livingstone, London, United Kingdom.
- 7.Gilligan, P. H. 1991. Microbiology of airway disease in patients with cystic fibrosis. Clin. Microbiol. Rev. 4:35-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Govan, J. R. W., J. E. Hughes, and P. Vandamme. 1996. Burkholderia cepacia: medical, taxonomic and ecological issues. J. Med. Microbiol. 45:395-407. [DOI] [PubMed] [Google Scholar]
- 9.Ilses, A. M., I. Maclusky, M. Corey, R. Gold, C. Probey, P. Fleming, and H. Levison. 1984. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J. Pediatr. 86:206-210. [DOI] [PubMed] [Google Scholar]
- 10.Kadurugamuwa, J. L., and T. J. Beveridge. 1998. Delivery of the non-membrane-permeative antibiotic gentamicin into mammalian cells by using Shigella flexneri membrane vesicles. Antimicriob. Agents Chemother. 42:1476-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadurugamuwa, J. L., and T. J. Beveridge. 1996. Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: conceptually new antibiotics. J. Bacteriol. 178:2767-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadurugamuwa, J. L., and T. J. Beveridge. 1995. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J. Bacteriol. 177:3998-4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadurugamuwa, J. L., J. S. Lam, and T. J. Beveridge. 1993. Interaction of gentamicin with the A band and B band lipopolysaccharides of Pseudomonas aeruginosa and its possible lethal effect. Antimicrob. Agents Chemother. 37:715-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewenza, S., B. Conway, E. P. Greenberg, and P. A. Sokol. 1999. Quorum sensing in Burkholderia cepacia: identification of the LuxRI homologs CepRI. J. Bacteriol. 181:748-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lightfoot, J., and J. S. Lam. 1991. Molecular cloning of genes involved with expression of A-band lipopolysaccharide, an antigenically conserved form, in Pseudomonas aeruginosa. J. Bacteriol. 173:5624-5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacDonald, K. L., and T. J. Beveridge. 2002. Bacteriolytic effect of gentamicin-induced membrane vesicles from Pseudomonas aeruginosa PAO1 on gram-positive bacteria. Can. J. Microbiol. 48:810-820. [DOI] [PubMed] [Google Scholar]
- 17.Moore, R. A., and R. E. W. Hancock. 1986. Involvement of outer membrane of Pseudomonas cepacia in aminoglycoside and polymyxin resistance. Antimicrob. Agents Chemother. 30:923-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riedel, K., M. Hentzer, O. Geisenberger, B. Huber, A. Steidle, H. Wu, N. Høiby, M. Givskov, S. Molin, and L. Eberl. 2001. N-Acylhomoserine-lactone-mediated communication between Pseudomonas aeruginosa and Burkholderia cepacia in mixed biofilms. Microbiology 147:3249-3262. [DOI] [PubMed] [Google Scholar]
- 19.Saini, L. S., S. B. Galsworthy, M. A. John, and M. A. Valvano. 1999. Intracellular survival of Burkholderia cepacia complex isolates in the presence of macrophage cell activation. Microbiology 145:2465-3467. [DOI] [PubMed] [Google Scholar]
- 20.Sajjan, U. S., L. Sun, R. Goldstein, and J. F. Forstner. 1995. Cable (Cbl) type II pili of cystic fibrosis-associated Burkholderia (Pseudomonas) cepacia: nucleotide sequence of the cblA major subunit pilin gene and novel morphology of the assembled appendage fibers. J. Bacteriol. 177:1030-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh, P. K., A. L. Schaefer, M. R. Parsek, T. O. Moninger, M. J. Welsh, and E. P. Greenberg. 2000. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407:762-764. [DOI] [PubMed] [Google Scholar]
- 22.Speert, D. P., D. Henry, P. Vandamme, M. Corey, and E. Mahenthiralingam. 2002. Epidemiology of Burkholderia cepacia in patients with cystic fibrosis, Canada. Emerg. Infect. Dis. 8:181-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Speert, D. P., M. Bond, and R. C. Woodman. 1994. Infection with Pseudomonas cepacia in chronic granulomatous disease: role of nonoxidative killing by neutrophils in host defense. J. Infect. Dis. 170:1524-1531. [DOI] [PubMed] [Google Scholar]
- 24.Wigfield, S. M., G. P. Rigg, M. Kavari, A. K. Webb, R. C. Matthews, and J. P. Burnie. 2002. Identification of an immunodominant drug efflux pump in Burkholderia cepacia. J. Antimicrob. Chemother. 49:619-624. [DOI] [PubMed] [Google Scholar]
- 25.Wilkinson, S. G., and T. L. Pitt. 1995. Burkholderia (Pseudomonas) cepacia: pathogenicity and resistance. Rev. Med. Microbiol. 6:1-17. [Google Scholar]
- 26.Yaron, S., G. L. Kolling, L. Simon, and K. R. Matthews. 2000. Vesicle-mediated transfer of virulence genes from Escherichia coli O157:H7 to other enteric bacteria. Appl. Environ. Microbiol. 66:4414-4420. [DOI] [PMC free article] [PubMed] [Google Scholar]