Abstract
During the last decade some studies have shown that the area under the curve (AUC)/MIC ratio is the pharmacodynamic index that best predicts the efficacies of quinolones, while other studies suggest that the predictive value of the peak concentration/MIC (peak/MIC) ratio is superior to the AUC/MIC ratio in explaining clinical and microbiological outcomes. In classical fractionated dose-response studies with animals, it is difficult to differentiate between the AUC/MIC ratio and the peak/MIC ratio because of colinearity. Three different levofloxacin and ciprofloxacin dosing regimens were studied in a neutropenic mouse pneumonia model. The different regimens were used with the aim of increasing the AUC/MIC ratio without changing the peak/MIC ratio and vice versa. The first regimen (RC) consisted of daily doses of 5 up to 160 mg/kg of body weight divided into one, two, or four doses. In the second regimen (R0), mice were given 1.25 mg/kg every hour from 1 to 23 h, while the dose given at 0 h was 2.5, 5, 10, 20, 40, or 80 mg/kg. In the third regimen (R11), mice also received 1.25 mg/kg every hour from 0 to 23 h; but in addition, they also received 2.5, 5, 10, 20, 40, or 80 mg/kg at 11 h. The level of protein binding was also evaluated. The results indicate that the unbound fraction (fu) was concentration dependent for both levofloxacin and ciprofloxacin and ranged from approximately 0.67 to 0.88 for both drugs between concentrations of 0.5 and 80 mg/liter. The relationships between the AUC/MIC ratio and the number of CFU were slightly better than those between the peak/MIC ratio and the number of CFU. There was no clear relationship between the amount of time that the concentration remained above the MIC and effect (R2 < 0.1). For both drugs, the peak/MIC ratio that resulted in a 50% effective concentration was lower for the R0 and R11 dosing regimens, indicating the importance of the AUC/MIC ratio. The same was true for the static doses. Survival studies showed that for mice treated with the low doses the rate of survival was comparable to that for the controls, but with the higher doses the rate of survival was better for mice receiving the R0 regimen. We conclude that for quinolones the AUC/MIC ratio best correlates with efficacy against pneumococci and that the effect of the peak/MIC ratio found in some studies could be partly explained by concentration-dependent protein binding.
When anti-infective agents are investigated, a measure of the potency of the drug toward the infecting pathogen is required, in addition to a measure of drug exposure. During the last decade, pharmacokinetic and pharmacodynamic analyses of dose-response relationships have resulted in a wealth of information. With regard to the quinolones, various studies have shown that the area under the curve (AUC)/MIC ratio and in some cases the peak concentration/MIC (peak/MIC) ratio are predictive of efficacy in studies with in vitro pharmacokinetic models (18, 20, 21, 24) and animals (2, 4, 5, 10, 12, 28, 29) and in clinical studies (1, 13, 14, 17, 30).
Several controversies remain, however, and these are best exemplified by the recent discussion between Drusano et al. (11) and Schentag et al. (32, 33). While the latter group of investigators maintain the view that the AUC/MIC ratio is the best predictor of quinolone efficacy, the former group of investigators take a more conservative approach. Importantly, they attach considerable importance to the peak/MIC ratio, with the predictive value depending on the ratio itself.
One of the reasons for this controversy is the experimental basis of the arguments. For determination of the predictive pharmacokinetic and pharmacodynamic indices for efficacy in the classical experiment, increasing daily doses are administered to infected animals, with the doses divided and administered one or more times daily. These are referred to as dose fractionation studies (8, 19). The predictive value of each of the pharmacokinetic and pharmacodynamic indices, i.e., the time that the concentration remains above the (T > MIC), the peak/MIC ratio, and the AUC/MIC ratio, can then be determined. Although this approach is very valuable in sorting out whether a drug has a time-dependent or dose-dependent effect, it is less valuable in sorting out the difference between the predictive value of the peak/MIC ratio and the AUC/MIC ratio. There appear to be two main shortcomings. The first is that, because of the nature of the dose fractionation studies, a considerable correlation between the peak/MIC ratio and the AUC/MIC ratio exists within the range of responses. The second argument is even more important: if a daily dose is fractionated and administered two, four, or more times a day, the peak concentration for each of these administrations is identical. Thus, it is impossible to determine the additional value of the second or later peak concentration. Also, it is not possible to determine whether the timing of the peak concentration plays a role.
We therefore performed a series of alternative experiments to evaluate the predictive value of the peak/MIC ratio compared with that of the AUC/MIC ratio. Instead of the classical dose fractionation study with identical doses, increasing daily doses were given by administering one peak dose and a series of low doses. In that way, the AUC was increased without any effect on the peak concentration. In addition, the doses that resulted in a high peak concentration were administered either at the start of therapy (0 h) or after 11 h in order to evaluate the relationship between the timing of the peak concentration and efficacy, in both cases during repetitive administrations of low doses of a quinolone. We also looked at the effect of protein binding on the relationship between the pharmacokinetic and the pharmacodynamic indices and the effect.
MATERIALS AND METHODS
Bacteria, media, and antibiotics.
Experiments were performed with a virulent Streptococcus pneumoniae strain (serotype 3) isolated in our laboratory. The strain was kept in batches at −70°C in brain heart infusion broth with 15% glycerol. The numbers of CFU on Mueller-Hinton (MH) agar plates supplemented with 5% sheep blood were counted. All media were obtained from Oxoid (Milan, Italy). Levofloxacin and ciprofloxacin were obtained commercially from Aventis (Lainate, Italy) and Bayer (Milan, Italy), respectively. Stock solutions were prepared as recommended by the manufacturers.
MICs and MBCs.
MICs and MBCs were determined three times by two different laboratories by standard microdilution procedures with geometric twofold serial dilutions in Todd-Hewitt broth according to the guidelines of NCCLS (27) and by E-test (AB-Biodisk, Sweden).
Mouse preparation and infection.
Six-week-old specific-pathogen-free female C57BL/6 mice (weight, 23 to 27 g) were obtained from Charles River (Calco, Italy). The mice were rendered neutropenic (100 neutrophils/mm3) by the intraperitoneal injection of two doses of cyclophosphamide 4 days (150 mg/kg of body weight) and 1 day (100 mg/kg) before the experiment.
Inocula of S. pneumoniae for infection were obtained by overnight incubation in brain heart infusion broth, washing of the cells twice by centrifugation at approximately 3,000 × g for 10 min in saline, and resuspension to a final inoculum of 107 CFU/ml in saline. Pneumonia was induced in propofol-anesthetized mice by applying approximately 3 × 106 CFU intratracheally. The inoculum was checked by plating 10-fold dilutions on MH agar plates. The lower limit of detection was 102 CFU in all experiments.
Antimicrobial treatment.
Antimicrobial treatment was started 10 h after inoculation by administration of 0.2 ml of antimicrobial solution subcutaneously, with the concentration administered depending on the dose. Each dosing group consisted of four to six mice. The animals were killed after 24 h for determination of CFU counts. The lungs were excised and homogenized in 10 ml of saline by using a Polytron homogenizer (Kinematica, Lucerne, Switzerland). Samples were plated on MH agar plates in 10-fold dilutions, and the number of CFU was used to backcalculate the number of CFU originally present in the lungs. Control mice were killed for organism quantification at the following times: just after intratracheal inoculation (n = 3), just before drug treatment for pneumonia (n = 6 to 8), and 24 h after the onset of therapy (n = 2). Three different dosing regimens were studied for each of the two drugs. The first regimen (RC regimen) consisted of daily doses of, 5, 10, 20, 40, 80, and 160 mg/kg divided into one, two, or four doses. In the second regimen (R0 regimen), mice were given 1.25 mg/kg every hour from 1 to 23 h, while the dose administered at 0 h was 2.5, 5, 10, 20, 40, or 80 mg/kg. In the third regimen (R11 regimen), mice also received 1.25 mg/kg every hour from 0 to 23 h but, in addition, also received 2.5, 5, 10, 20, 40, or 80 mg/kg at 11 h.
Survival studies.
Survival studies were carried out with mice receiving either the R0 or the R11 regimen, with 15 mice per dosing group. The dosing regimens were the same as those during the efficacy studies involving CFU counts. The mice were monitored for 10 days.
All animals used in this study were housed in accordance with the regulations of the Home Office of the Italian Government.
Drug pharmacokinetics.
Single-dose pharmacokinetic studies were performed on the day of antimicrobial treatment with samples from lung-infected mice receiving the complete range of individual doses used. In addition, samples were obtained from mice receiving the 1.25-mg/kg doses ever hour during steady state, which was achieved at 7 h. For each group of five to six mice receiving the individual doses of each drug examined, samples were obtained at 10- to 60-min intervals over 8 h. Blood was obtained by decapitation, collected and placed into heparinized tubes, and centrifuged for 10 min; plasma drug levels were determined with a high-pressure liquid chromatograph (Shimadzu, Milan, Italy), as reported previously (34). Standard samples were prepared in pooled normal mouse serum. For both drugs, the method was linear for concentrations ranging from 0.05 to 5.0 μg per ml. The intraday and interday coefficients of variation for levofloxacin ranged from 0.5 to 5.5% and 5.6 to 13.3%, respectively, over the range of concentrations detected. For ciprofloxacin, these values were 0.5 to 5.5% and 5.2 to 12.3%, respectively. Since the method used determined the concentrations of total drug, values for the unbound fraction (fu) of drug were calculated by using protein binding values, which were determined as follows: pooled mouse serum was spiked with levofloxacin or ciprofloxacin to final total concentrations ranging from 0.5 to 80 mg/liter. The free fractions of the drugs were then obtained by ultrafiltration by using the Centrifree micropartition system (Amicon Division, W. R. Grace & Co., Beverly, Mass.). The samples were equilibrated for 30 min at 37°C and then centrifuged at 2,500 × g for 20 min at 25°C. The free concentrations in the ultrafiltrate and the total concentrations in serum were determined by high-pressure liquid chromatography analysis, as described above.
Pharmacokinetic and statistical analyses.
The relationship between concentration and fu was described by the Boltzmann equation (Graphpad Prism, San Diego, Calif.). The values for the pharmacokinetic parameters were estimated by using both the total concentrations and the free fraction concentrations by using MWPharm (Mediware, Groningen, The Netherlands) and Winnonlin (Pharsight Corp., Mountain View, Calif.) software and a one-compartment open model with the lag time. Noncompartmental analysis was used to confirm the results. Pharmacodynamic index values (AUC/MIC ratio, peak/MIC ratio, and T > MIC) were calculated over a period of 24 h (26). Pharmacokinetic and pharmacodynamic index-response curves were fit to the CFU data by using a sigmoid maximum-effect (Emax) model with a variable slope (Graphpad Prism) to evaluate the impact of the dosing interval on efficacy. The static pharmacokinetic-pharmacodynamic index was calculated by substitution of the inoculum at the start of therapy in the Emax model. Survival was evaluated by the Lifetest procedure of the SAS program package (31). After the dosing regimens were tested for homogeneity over all strata, the results for the corresponding dosing regimens with the same dose at 0 and 11 h (the R0 and R11 regimens) were compared with each other and with those for the control regimen by the Wilcoxon test and the log-rank test.
RESULTS
MICs and MBCs.
The MIC of ciprofloxacin was 0.7 mg/liter, and the MIC of levofloxacin was 1 mg/liter. The MBCs of both drugs were 2 mg/liter.
Pharmacokinetic studies.
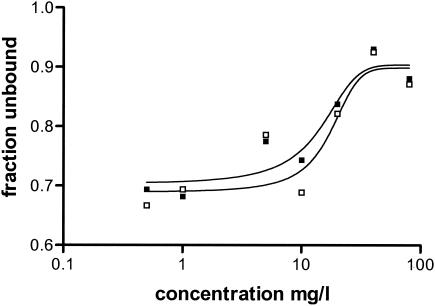
The fu was concentration dependent for both levofloxacin and ciprofloxacin and ranged from approximately 0.67 to 0.88 for both drugs between concentrations of 0.5 and 80 mg/liter. The relationship between fu and concentration could be described by the Boltzmann equation; the R2 values were 0.82 and 0.88, respectively (Fig. 1). Because of the concentration-dependent protein binding, the parameter estimates obtained were used to convert total concentrations in plasma to the free fraction values, and those data were directly fit to the pharmacokinetic models. Alternatively, the pharmacokinetic model was fit to total concentrations. The values of the pharmacokinetic parameters were linear for both levofloxacin and ciprofloxacin over the dosing range between 10 and 80 mg/kg. Higher doses resulted in a slightly longer half-life due to a larger estimated volume of distribution (V). For the unbound fraction, the values of V were 1.53 and 2.16 liters/kg for the 20-mg/kg doses of levofloxacin and ciprofloxacin, respectively, and the elimination half-lives were 0.83 and 0.69 h, respectively. The R2 values for the fits were >0.90 in all cases.
FIG. 1.
Percentage of free drug as a function of the total concentration. □, fu of levofloxacin; ▪, fu of ciprofloxcin.
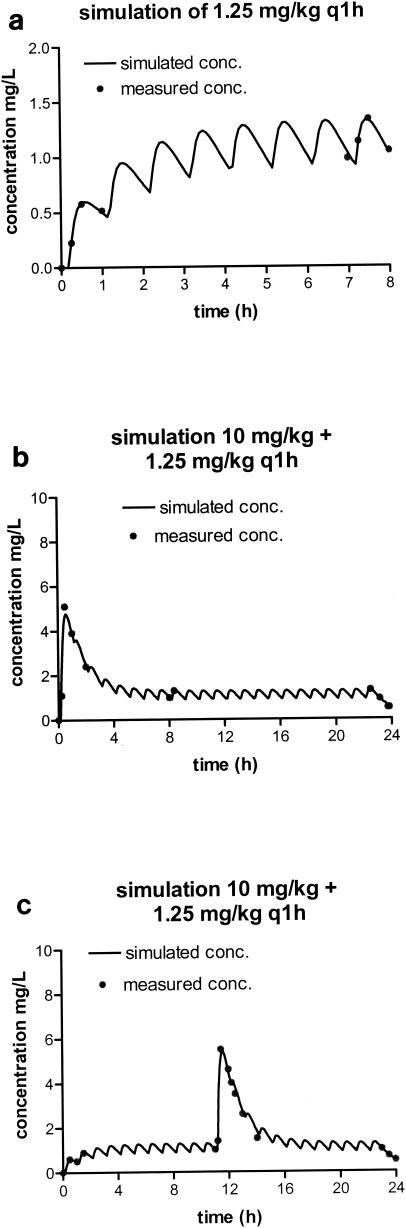
Figure 2 shows the fit of the pharmacokinetic data and simulations of the pharmacokinetic profiles for the levofloxacin regimens when the drug was given at 1.25 mg/kg every hour. There was considerable accumulation of levofloxacin over the first few doses (Fig. 2a). This has consequences for the peak values obtained with increasing doses at 0 and 11 h; peak levels were slightly higher at 11 h. The results of the simulations are shown in Fig. 1b and c. The impact on the peak/MIC ratio is dependent on the dose given: the ratio almost doubles with the lowest dose, while the effect with the high doses is hardly significant. The profile for ciprofloxacin was similar, although the level of accumulation was slightly less because of its shorter half-life (data not shown).
FIG. 2.
Simulated concentration-time profiles of levofloxacin (free fraction) based on derived pharmacokinetic parameters. (a) Simulated and measured concentrations after administration of the first dose and during steady state for the 1.25-mg/kg dosing regimen; (b and c) simulated concentration-time profiles for the R0 and R11 regimens, respectively, after the administration of a single dose of 10 mg/kg.
Pharmacodynamic studies. (i) Conventional dose fractionation and effects of peak/MIC ratio on total drug concentration.
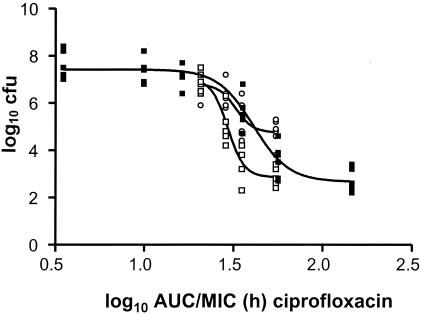
Figure 3 shows the relationship between the number of CFU and the AUC/MIC ratio for levofloxacin for the conventional dose fractionation studies (RC regimen), the R0 regimen, and the R11 regimen for total drug. The relationship for ciprofloxacin was virtually identical (data not shown). The relationships shown in Fig. 3 are for the regimen given in a conventional manner, with increasing doses given at various intervals. The relationship between the AUC/MIC ratio and the number of CFU is similar to that between the peak/MIC ratio and the number of CFU. There was no clear relationship between T > MIC and effect (R2 < 0.1). The correlations between the logarithms of AUC and peak values were 0.87 and 0.86 for ciprofloxacin and levofloxacin, respectively (P < 0.001). From Fig. 3 it can be observed that with lower AUC/MIC ratios the conventional regimen is more efficacious than the R0 regimen and the R11 regimen, while with the higher doses, the R0 regimen was particularly more efficacious by use of the same AUC/MIC ratio. Since this may be related to the peak/MIC ratio of the specific regimens, this “crossing point” was determined (Fig. 3, arrow). The peak/MIC ratios of the R0 regimen at this crossing point were 7.1 for levofloxacin and 9.4 for ciprofloxacin.
FIG. 3.
Relationship between the AUC/MIC ratio and effect for levofloxacin for total drug and the three different regimens. Doses were given every 24, 12, or 6 h (RC regimen) (▪), every 24 h at 0 h and at 1.25 mg/kg every 1 h (R0 regimen) (□), and every 24 h at 11 h and at 1.25 mg/kg every 1 h (R11 regimen) (○). The arrow indicates the peak/MIC ratio where the R0 regimen becomes more efficacious with increasing AUC/MIC ratios.
(ii) Conventional dose fractionation and peak/MIC ratio effects for free fraction of drug.
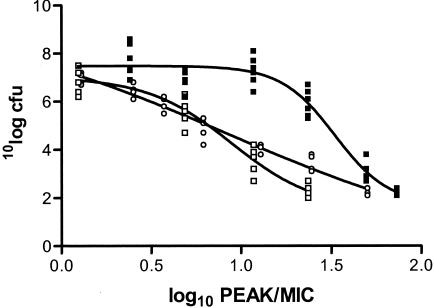
Figure 4 shows the relationships between the peak/MIC ratio and effect for levofloxacin and the R0 and R11 regimens for the unbound fraction of levofloxacin. Figure 4 also includes the results obtained with the RC regimen with dosing once every 24 h. Thus, at the same peak concentration the AUC for the RC regimen was lower than the AUCs for the two regimens that included dosing at 1.25 mg/kg every 1 h. Two important conclusions can be drawn from the results presented in Fig. 4. The first one is that the effects of the R0 and R11 regimens have resulted in a shift of the values to the left and/or below those for the RC regimen. This indicates that the higher AUC/MIC ratios (and the same peak concentrations) for the R0 and R11 regimens add to the overall effect. The second conclusion is that the shapes of the curves for the R0 and R11 regimens are significantly different from and have shallower slopes than the curve for the RC regimen, with the curve for the peak concentration at 11 h (R11 regimen) having a shallower slope than the curve for the peak concentration at 0 h (R0 regimen). These conclusions are substantiated by the data presented in Table 1, which shows the pharmacokinetic-pharmacodynamic index values that result in steady state and the doses of antibiotics required to inhibit bacterial growth by 50% (EI50). For both drugs, the peak/MIC ratio that resulted in EI50 was lower for the R0 and R11 dosing regimens, indicating the importance of the AUC/MIC ratio (for the RC regimen versus the R0 and R11 regimens, the same peak/MIC ratio includes a higher AUC/MIC ratio). The same is true for the static doses.
FIG. 4.
Relationship between the peak/MIC ratio and effect for levofloxacin for three different regimens for unbound fraction of the drug. For the RC regimen, only the results for regimens with dosing every 24 h are shown. ▪, RC regimen; □, R0 regimen; ○, R11 regimen. The data on the y axis are log10 CFU.
TABLE 1.
R2 values and EI50, Hill slope, and static index estimates from Emax model with a variable slope for unbound fraction of drugs
| Drug and regimen | AUC/MIC ratio
|
Peak/MIC ratio
|
||||||
|---|---|---|---|---|---|---|---|---|
| EI50 (mg/kg) | R2 | Hill slope | Static index (h) | EI50 (mg/kg) | R2 | Hill slope | Static index (h) | |
| Levofloxacin | ||||||||
| RC | 46.4 (41.6-51.8)a | 0.91 | −2.8 (−3.6 to −2.1) | 31.6 | 22.8 (7.4-70.0) | 0.78 | −1.0 (−1.7 to −0.2) | 7.2 |
| RC firstb | 40.9 (35.5-47.2) | 0.94 | −3.5 (−4.9 to −2.1) | 28.2 | 33.1 (25.4-43.0) | 0.94 | −2.9 (−4.4 to −1.4) | 20.9 |
| R0 | 37.7 (36.7-39.2) | 0.94 | −11.8 (−16.6 to −7.1) | 31.6 | 8.9 (6.0-13.4) | 0.94 | −1.8 (−3.0 to −0.7) | 3.2 |
| R11 | —c | — | — | — | 8.5 (2.5-29.6) | 0.96 | −0.6 (−1.6 to 0.4) | 2.5 |
| Ciprofloxacin | ||||||||
| RC | 44.1 (40.5-48.1) | 0.91 | −3.5 (−4.5 to −2.6) | 33.1 | 11.3 (9.1-14.1) | 0.79 | −2.0 (−2.9 to −1.1) | 5.2 |
| RC first | 40.7 (36.1-46.0) | 0.91 | −4.4 (−6.5 to −2.2) | 30.9 | 25.3 (16.6-38.7) | 0.92 | −2.1 (−3.4 to −0.8) | 10.5 |
| R0 | 29.4 (27.3-31.6) | 0.88 | −9.4 (−16.2 to −2.6) | 24.5 | 3.1 (2.4-3.7) | 0.88 | −3.8 (−6.9 to −0.8) | 1.5 |
| R11 | 32.5 (27.7-38.0) | 0.63 | −9.0 (−20.9 to −2.9) | 28.8 | 4.7 (3.3-3.6) | 0.63 | −4.4 (−11.1 to −2.4) | 2.6 |
The values in parentheses are 95% confidence intervals.
Data for the first dose only of the RC regimen.
—, no fit could be obtained.
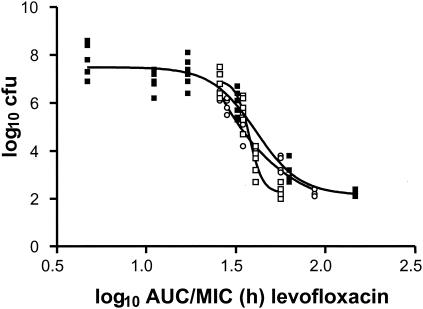
Figures 5 and 6 show the relationships between the AUC/MIC ratio and effect for levofloxacin and ciprofloxacin for unbound drug, respectively. Since with the same AUC/MIC ratio the peak concentration achieved with the RC regimen is higher, this apparently has the effect that the curves for the R0 and R11 regimens display steeper slopes, as expressed by higher Hill coefficients (Table 1). Similar to the results shown in Fig. 3 for total drug, it seems that there is an AUC/MIC ratio at which the effect of the R0 regimen is inferior but that at a certain AUC/MIC ratio the effect becomes superior. The value cannot be determined, however, and the effect is almost negligible and not significant. This is better observed for ciprofloxacin, for which there was no crossing point at all. Therefore, it is concluded that the AUC/MIC ratio itself is possibly more important than the peak/MIC ratio. In fact, Fig. 5 and 6 indicate that maintenance of the concentration above the MIC for some time is important for quinolones.
FIG. 5.
Relationship between the AUC/MIC ratio and effect for levofloxacin for three different regimens for unbound fraction of the drug. Doses were given every 24 h (RC regimen) (▪), every 24 h at 0 h and at 1.25 mg/kg every 1 h (R0 regimen), and every 24 h at 11 h and at 1.25 mg/kg every 1 h (R11 regimen) (○).
FIG. 6.
Relationship between the AUC/MIC ratio and effect of ciprofloxacin for three different regimens for unbound fraction of the drug. Doses were given every 24 h (RC regimen) (▪), every 24 h at 0 h and at 1.25 mg/kg every 1 h (R0 regimen) (□), and every 24 h at 11 h and at 1.25 mg/kg every 1 h (R11 regimen) (○).
Effect of timing of peak concentration.
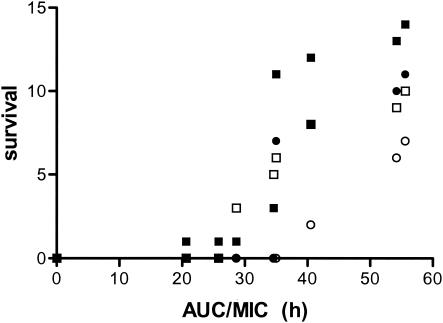
As stated above, a peak concentration at 11 h results in an effect curve with a shallower slope (expressed as a smaller Hill coefficient) compared with the slope for the curve for the peak concentration when the dose is given at the start of therapy. The maximum effect is therefore reached at higher doses. The results of the survival studies support this conclusion. For mice receiving the low doses survival was comparable to that for the controls, but among mice receiving the higher doses survival was better for those in the R0 group. The results for ciprofloxacin were comparable. Figure 7 shows the relationship between the AUC of the free fraction of the drugs (both levofloxacin and ciprofloxacin) and survival at day 6 and day 10 for the R0 and R11 regimens. Survival on day 6 is also included because this was the first day that all animals in the control group had died and therefore shows a good discrimination between the various regimens. The efficacy of the R0 regimen is clearly superior to that of the R11 regimen.
FIG. 7.
Relationship between AUC/MIC ratio for the nonprotein bound fraction of levofloxacin and ciprofloxacin for the R0 and R11 regimens on day 6 and day 10. ○, R0 regimen on day 10; ○, R11 regimen on day 10; ▪, R0 regimen on day 6; □, R11 regimen on day 6.
DISCUSSION
In the present study, a pneumonia model was used to expose a strain of S. pneumoniae to drugs at a range of AUC/MIC ratios without altering the peak/MIC ratios, and vice versa. Traditional analysis (Fig. 3) supports the conclusions from previous studies with various quinolones that the AUC/MIC ratio correlates well with efficacy, as does the peak/MIC ratio. However, because of the way in which dose fractionation studies are usually performed, it is difficult to separate out the relative contributions of the AUC/MIC ratio and the peak/MIC ratio to the overall effect. The correlation between these two pharmacodynamic indices is relatively large. In an analysis of three dosing regimens of gemifloxacin, MacGowan and colleagues (22, 23) found a correlation coefficient of 0.77, emphasizing this specific issue. In this study, it was demonstrated that the AUC/MIC ratio had a more beneficial effect than the peak/MIC ratio. This was shown by the additional efficacy of low frequent dosing with levofloxacin and ciprofloxacin, which resulted in a higher AUC without impeding the peak/MIC ratio. In addition, both the results of the CFU counts and the survival studies indicate that the R0 regimen was superior to the R11 regimen at comparable AUCs. The R0 regimen is probably superior because the AUC is higher earlier and the peak concentration is achieved earlier, so that the mouse is subjected to a higher inoculum for a shorter period of time within the first 12 h. Moreover, a selection of less susceptible clones could be involved. Taken together, it may be concluded that both the AUC/MIC ratio and the peak/MIC ratio correlate with efficacy but that the AUC/MIC ratio shows a better correlation. Moreover, it seems that the administration of doses that sustain concentrations to obtain a certain AUC/MIC ratio is superior to the administration of one high dose. The latter phenomenon is possibly related to the relatively short half-lives of quinolones in mice. However, if the effects of the regimens of administration every 6 h are compared with those of the regimens of administration every 12 and 24 h in the conventional dose fractionation, the regimens of administration every 6 h are slightly less effective than the regimens of administration every 12 and 24 h at the same AUC/MIC (data not shown).
According to the peak/MIC ratios of total drug, the R0 regimen becomes superior at increasing concentrations, and the peak/MIC ratios of total drug are of the same magnitude as the ratios found in in vitro pharmacokinetic models of infection. In a study by Blaser et al. (6) in which they simulated the concentration-time profiles of enoxacin in humans, a peak/MIC ratio of at least 8 was needed to prevent the regrowth of bacteria. In a similar model, Marchbanks et al. (24a) showed that once-daily dosing of ciprofloxacin yielded a better outcome than more frequent dosing. In a human trial, a peak/MIC ratio of 12.2 was found (30).
The conclusions from our experiments could explain the discrepant results found by various investigators in human trials. Three human trials performed to evaluate the efficacies of quinolones found a good relationship between the AUC/MIC ratio and effect (13, 14, 17). In a fourth trial, the only prospective study that has been conducted (28), there was a relatively good correlation between the AUC/MIC ratio and effect, but the relationship between the peak/MIC ratio and effect was slightly superior. In addition, classification and regression tree analysis in that study showed that there was a significantly better effect if the peak/MIC ratio was more than 12.2. Since no data on protein binding were provided, the concentration dependency of protein binding could be an explanation.
One of the problems with the way in which a dose fractionation study is usually analyzed is that an R2 value is determined for the fit of the model used; this is usually an Emax model. The pharmacokinetic-pharmacodynamic index that best correlates with the outcome data is then assumed to be the index that best predicts the effect. However, this method of analysis assumes not only that the data are homoscedastic but also that the importance of each datum point is equal. When we are treating patients, however, we are not necessarily interested in the best correlation between index and outcome but are primarily interested in the value of the index that is needed to ensure at least a good outcome. Thus, although the overall correlation of effect with the AUC/MIC ratio may be better as shown by a higher R2 value, this does not necessarily indicate that one of the other pharmacokinetic and pharmacodynamic indices has more predictive value within the range of interest. The results of the present study underline the fact that a good correlation does not necessarily lead to a better outcome but that a more detailed analysis is needed to determine the predictive value of a pharmacokinetic-pharmacodynamic index.
Because it has clearly been shown in many studies (3, 7, 9, 25) that it is only the free, unbound fraction of the drug that is active, all data and modeling were done with both the total and the free fractions of the drugs. To determine the free fraction, the concentrations of both bound and unbound drug were measured. To our surprise, it appeared that the free fraction was dependent on the concentration. Reports on the levels of protein binding of these drugs are hard to find in the literature; we found protein binding findings only in the recent veterinary literature (15, 16). It did, however, have an effect on the results and the conclusions. Because of the concentration dependence of protein binding, we chose to analyze and model the data for both the free fraction of the drug and the total drug. Although the general conclusions were more or less similar, they did differ in one aspect, in that there was a more pronounced effect of the peak/MIC ratio when data for the total drug concentration were used. This might be because there is a relatively larger fraction of free drug at peak concentrations than at lower concentrations, and this larger fraction of free drug may thus have a greater effect because at the same AUC a higher peak concentration will result in a larger fraction of free drug. Thus, it is not the peak concentration itself which leads to a better outcome but the fact that the AUC of the free fraction is larger if the peak concentration is higher. This could partly explain why peak concentrations have been given importance in explaining outcome. Since we are not aware of protein binding studies over a whole concentration range with samples from humans, this remains open to question but should be looked at in further studies.
Acknowledgments
This work was supported by a grant from MURST (grant protocol 2001058114, 2001).
REFERENCES
- 1.Ambrose, P. G., D. M. Grasela, T. H. Grasela, J. Passarell, H. B. Mayer, and P. F. Pierce. 2001. Pharmacodynamics of fluoroquinolones against Streptococcus pneumoniae in patients with community-acquired respiratory tract infections. Antimicrob. Agents Chemother. 45:2793-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andes, D. R., and W. A. Craig. 1998. Pharmacodynamics of fluoroquinolones in experimental models of endocarditis. Clin. Infect. Dis. 27:47-50. [DOI] [PubMed] [Google Scholar]
- 3.Bakker-Woudenberg, I. A., J. C. van den Berg, T. B. Vree, A. M. Baars, and M. F. Michel. 1985. Relevance of serum protein binding of cefoxitin and cefazolin to their activities against Klebsiella pneumoniae pneumonia in rats. Antimicrob. Agents Chemother. 28:654-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedos, J. P., E. Azoulay-Dupuis, P. Moine, M. Muffat-Joly, B. Veber, J. J. Pocidalo, and E. Vallee. 1998. Pharmacodynamic activities of ciprofloxacin and sparfloxacin in a murine pneumococcal pneumonia model: relevance for drug efficacy. J. Pharmacol. Exp. Ther. 286:29-35. [PubMed] [Google Scholar]
- 5.Bedos, J. P., V. Rieux, J. Bauchet, M. Muffat-Joly, C. Carbon, and E. Azoulay-Dupuis. 1998. Efficacy of trovafloxacin against penicillin-susceptible and multiresistant strains of Streptococcus pneumoniae in a mouse pneumonia model. Antimicrob. Agents Chemother. 42:862-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaser, J., B. B. Stone, M. C. Groner, and S. H. Zinner. 1987. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob. Agents Chemother. 31:1054-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cars, O. 1990. Pharmacokinetics of antibiotics in tissues and tissue fluids: a review. Scand. J. Infect. Dis. Suppl. 74:23-33. [PubMed] [Google Scholar]
- 8.Craig, W. A. 1998. Choosing an antibiotic on the basis of pharmacodynamics. Ear Nose Throat J. 77(Suppl. 6):7-11. [PubMed] [Google Scholar]
- 9.Craig, W. A., and S. C. Ebert. 1989. Protein binding and its significance in antibacterial therapy. Infect. Dis. Clin. N. Am. 3:407-414. [PubMed] [Google Scholar]
- 10.Drusano, G. L., D. E. Johnson, M. Rosen, and H. C. Standiford. 1993. Pharmacodynamics of a fluoroquinolone antimicrobial agent in a neutropenic rat model of Pseudomonas sepsis. Antimicrob. Agents Chemother. 37:483-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drusano, G. L., S. L. Preston, R. C. Owens, Jr., and P. G. Ambrose. 2001. Fluoroquinolone pharmacodynamics. Clin. Infect. Dis. 33:2091-2096. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez, J., J. F. Barrett, L. Licata, D. Amaratunga, and M. Frosco. 1999. Comparison of efficacies of oral levofloxacin and oral ciprofloxacin in a rabbit model of a staphylococcal abscess. Antimicrob. Agents Chemother. 43:667-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forrest, A., D. E. Nix, C. H. Ballow, T. F. Goss, M. C. Birmingham, and J. J. Schentag. 1993. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob. Agents Chemother. 37:1073-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forrest, A., S. Chodosh, M. A. Amantea, D. A. Collins, and J. J. Schentag. 1997. Pharmacokinetics and pharmacodynamics of oral grepafloxacin in patients with acute bacterial exacerbations of chronic bronchitis. J. Antimicrob. Chemother. 40(Suppl. A):45-57. [DOI] [PubMed] [Google Scholar]
- 15.Friis, C. 1993. Penetration of danofloxacin into the respiratory tract tissues and secretions of calves. Am. J. Vet. Res. 54:1122-1127. [PubMed] [Google Scholar]
- 16.Greco, C. 2003. Tissue cages in calves for studies on pharmacokinetic/pharmcodynamic relationships of antimicrobials. Ph.D. thesis. Swedish University of Agricultural Sciences, Uppsala, Sweden.
- 17.Highet, V. S., A. Forrest, C. H. Ballow, and J. J. Schentag. 1999. Antibiotic dosing issues in lower respiratory tract infection: population-derived area under inhibitory curve is predictive of efficacy. J. Antimicrob. Chemother. 43(Suppl. A):55-63. [DOI] [PubMed] [Google Scholar]
- 18.Lacy, M. K., W. Lu, X. Xu, P. R. Tessier, D. P. Nicolau, R. Quintiliani, and C. H. Nightingale. 1999. Pharmacodynamic comparisons of levofloxacin, ciprofloxacin, and ampicillin against Streptococcus pneumoniae in an in vitro model of infection. Antimicrob. Agents Chemother. 43:672-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leggett, J. E., S. Ebert, B. Fantin, and W. A. Craig. 1990. Comparative dose-effect relations at several dosing intervals for beta-lactam, aminoglycoside and quinolone antibiotics against gram-negative bacilli in murine thigh-infection and pneumonitis models. Scand. J. Infect. Dis. Suppl. 74:179-184. [PubMed] [Google Scholar]
- 20.Lister, P. D., and C. C. Sanders. 1999. Pharmacodynamics of levofloxacin and ciprofloxacin against Streptococcus pneumoniae. J. Antimicrob. Chemother. 43:79-86. [DOI] [PubMed] [Google Scholar]
- 21.Lister, P. D., and C. C. Sanders. 1999. Pharmacodynamics of trovafloxacin, ofloxacin, and ciprofloxacin against Streptococcus pneumoniae in an in vitro pharmacokinetic model. Antimicrob. Agents Chemother. 43:1118-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacGowan, A., and K. Bowker. 2002. Developments in PK/PD: optimising efficacy and prevention of resistance. A critical review of PK/PD in in vitro models. Int. J. Antimicrob. Agents 19:291-298. [DOI] [PubMed] [Google Scholar]
- 23.MacGowan, A. P., C. A. Rogers, H. A. Holt, M. Wootton, and K. E. Bowker. 2001. Pharmacodynamics of gemifloxacin against Streptococcus pneumoniae in an in vitro pharmacokinetic model of infection. Antimicrob. Agents Chemother. 45:2916-2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madaras-Kelly, K. J., B. E. Ostergaard, L. B. Hovde, and J. C. Rotschafer. 1996. Twenty-four-hour area under the concentration-time curve/MIC ratio as a generic predictor of fluoroquinolone antimicrobial effect by using three strains of Pseudomonas aeruginosa and an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 40:627-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a.Marchbanks, C. R., J. R. McKiel, D. H. Gilbert, N. J. Robillard, B. Painter, S. H. Zinner, and M. N. Dudley. 1993. Dose ranging and fractionation of intravenous ciprofloxacin against Pseudomonas aeruginosa and Staphylococcus aureus in an in vitro model of infection. Antimicrob. Agents Chemother. 37:1756-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattie, H. 1978. Tissue penetration of antibiotics. Pharmacokinetic aspects of tissue penetration. Scand. J. Infect. Dis. Suppl. 14:125-126. [PubMed] [Google Scholar]
- 26.Mouton, J. W., M. N. Dudley, O. Cars, H. Derendorf, and G. L. Drusano. 2002. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs. Int J. Antimicrob. Agents 19:355-358. [DOI] [PubMed] [Google Scholar]
- 27.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 28.Ng, W., I. Lutsar, L. Wubbel, F. Ghaffar, H. Jafri, G. H. McCracken, and I. R. Friedland. 1999. Pharmacodynamics of trovafloxacin in a mouse model of cephalosporin-resistant Streptococcus pneumoniae pneumonia. J. Antimicrob. Chemother. 43:811-816. [DOI] [PubMed] [Google Scholar]
- 29.Onyeji, C. O., K. Q. Bui, R. C. Owens, Jr., D. P. Nicolau, R. Quintiliani, and C. H. Nightingale. 1999. Comparative efficacies of levofloxacin and ciprofloxacin against Streptococcus pneumoniae in a mouse model of experimental septicaemia. Int. J. Antimicrob. Agents 12:107-114. [DOI] [PubMed] [Google Scholar]
- 30.Preston, S. L., G. L. Drusano, A. L. Berman, C. L. Fowler, A. T. Chow, B. Dornseif, V. Reichl, J. Natarajan, and M. Corrado. 1998. Pharmacodynamics of levofloxacin: a new paradigm for early clinical trials. JAMA 279:125-129. [DOI] [PubMed] [Google Scholar]
- 31.SAS Institute, Inc. 1993. SAS user's guide. SAS Institute, Inc., Cary, N.C.
- 32.Schentag, J. J., K. K. Gilliland, and J. A. Paladino. 2001. Reply. Clin. Infect. Dis. 33:2092-2096. [DOI] [PubMed] [Google Scholar]
- 33.Schentag, J. J., K. K. Gilliland, and J. A. Paladino. 2001. What have we learned from pharmacokinetic and pharmacodynamic theories? Clin. Infect. Dis. 32(Suppl. 1):S39-S46. [DOI] [PubMed] [Google Scholar]
- 34.Wong, F. A., S. J. Juzwin, and S. C. Flor. 1997. Rapid stereospecific high-performance liquid chromatographic determination of levofloxacin in human plasma and urine. J. Pharm. Biomed. Anal. 15:765-771. [DOI] [PubMed] [Google Scholar]