Abstract
The objective of this study was to determine the distribution profile of the novel endotoxin antagonist E5564 in plasma obtained from fasted human subjects with various lipid concentrations. Radiolabeled E5564 at 1 μM was incubated in fasted plasma from seven human subjects with various total cholesterol (TC) and triglyceride (TG) concentrations for 0.5 to 6 h at 37°C. Following these incubations, plasma samples were separated into their lipoprotein and lipoprotein-deficient fractions by ultracentrifugation and were assayed for E5564 radioactivity. TC, TG, and protein concentrations in each fraction were determined by enzymatic assays. Lipoprotein surface charge within control and phosphatidylinositol-treated plasma and E5564’s influence on cholesteryl ester transfer protein (CETP) transfer activity were also determined. We observed that the majority of E5564 was recovered in the high-density lipoprotein (HDL) fraction. We further observed that incubation in plasma with increased levels of TG-rich lipoprotein (TRL) lipid (TC and TG) concentrations resulted in a significant increase in the percentage of E5564 recovered in the TRL fraction. In further experiments, E5564 was preincubated in human TRL. Then, these mixtures were incubated in hypolipidemic human plasma for 0.5 and 6 h at 37°C. Preincubation of E5564 in purified TRL prior to incubation in human plasma resulted in a significant decrease in the percentage of drug recovered in the HDL fraction and an increase in the percentage of drug recovered in the TRL and low-density lipoprotein fractions. These findings suggest that the majority of the drug binds to HDLs. Preincubation of E5564 in TRL prior to incubation in normolipidemic plasma significantly decreased the percentage of drug recovered in the HDL fraction. Modifications to the lipoprotein negative charge did not alter the E5564 concentration in the HDL fraction. In addition, E5564 does not influence CETP-mediated transfer activity. Information from these studies could be used to help identify the possible components of lipoproteins which influence the interaction of E5564 with specific lipoprotein particles.
Inflammatory shock as a consequence of lipopolysaccharide (LPS) or endotoxin release from gram-negative bacteria remains a serious clinical concern (3). In humans, inflammatory responses to LPS result in the release of cytokines and other cell mediators from monocytes and macrophages, which can cause fever, shock, organ failure, and death (3). A number of different approaches have been investigated to try to treat and/or prevent the septic shock associated with infections caused by gram-negative bacteria, including blocking of one or more of the cytokines induced by LPS (14). Recently, novel amphipathic compounds E5531 and E5564 have been developed as direct antagonists of LPS at the LPS receptor, TLR4 (5).
Our laboratory has previously reported that the majority of E5531 (an analogue of E5564 which has chemical properties and pharmacological activity similar to those of E5531) associates with high-density lipoproteins (HDLs) upon incubation in human plasma at 37°C and that binding to lipoproteins is fast (within 5 min), with no measurable redistribution of E5531 between different lipoprotein fractions (21). Furthermore, it appears that increases in triglyceride (TG)-rich lipoprotein (TRL; which contains very low density lipoproteins and chylomicrons) and low-density lipoprotein (LDL) cholesterol, TG, and protein levels in plasma significantly increase the levels of TRL and LDL binding of E5531, while decreases in the levels of these fractions in plasma significantly increase the level of HDL binding of E5531. In addition, we have observed a rapid loss of the endotoxin antagonistic activity of E5531 upon binding to HDL but no such loss upon binding to LDL or TRL (16).
To date little is known about the lipoprotein distribution of E5564. Furthermore, studies have not been done to determine if changes in plasma lipid and lipoprotein concentrations, often observed in septic patients (1) who would be receiving this compound, would modify the plasma lipoprotein binding of E5564. Therefore, the objectives of this study were to determine the distribution profile of E5564 in plasma from patients with various plasma total and lipoprotein lipid and protein concentrations and the role of the HDL lipid composition on E5564 lipoprotein binding (i.e., to determine the HDL subfractions that dictate binding). Our hypothesis is that, like E5531, the majority of E5564 will be recovered in the HDL fraction.
MATERIALS AND METHODS
Reconstitution of [14C]E5564.
[14C]E5564 was reconstituted and diluted as previously described for E5531 (21).
Isolation and purification of CETP.
Cholesteryl ester (CE) transfer protein (CETP) was purified from human lipoprotein-deficient plasma, as described previously (13). Briefly, citrated human plasma was made lipoprotein deficient by the dextran-MnCl2 procedure of Burstein and coworkers (4). CETP was then partially purified by sequential chromatography on a phenyl-Sepharose and carboxymethyl cellulose gel (CM-52; Whatman, Inc., Chifton, N.J.). Partially purified CETP (1.05 mg of protein/ml), enriched 800-fold relative to the amount in lipoprotein-deficient plasma, was stored at 4°C in 0.01% disodium EDTA (pH 7.4). The carboxymethyl cellulose fraction of CETP was used in all experiments.
Radiolabeling of plasma lipoproteins.
Human LDL was labeled by the lipid dispersion technique described previously (13, 20). Briefly, human plasma was incubated with a lipid dispersion containing egg phosphatidylcholine, TG (5 mol%), and [3H]CE (12 mol%) at 37°C for 20 to 24 h in the presence of CETP and diethyl (p-nitrophenyl) phosphate. Then, the LDL fractions were isolated from the total lipoprotein precipitate by centrifugation, as described previously (20). LDL had a specific activity of 3.423 × 106 cpm/ml (1,352 cpm/μg of total cholesterol).
Lipid transfer assays.
The following protocol was used to assess the CETP activities within the different human plasma samples used in this study. Lipid (CE) transfer was performed within the lipoprotein-deficient plasma, as described previously (13, 20). Typically, 10 μg (total cholesterol) of radiolabeled donor ([3H]CE LDL) and unlabeled acceptor (HDL) are incubated with or without the CETP source (pH 7.4; which consisted of the different delipidated human plasma samples used in this study) for 90 min at 37°C. Lipid transfer between donor and acceptor lipoprotein is then quantitated by scintillation counting. The fraction of lipid and drug transferred (kt) was calculated as follows, as described by Pattnaik and Zilversmit (15): −ln[1 − (At/D0)], where D0 and At are the levels of radioactivity in the donor at time zero and in the acceptor at time t, respectively. The constant k is the fraction of label transferred per unit time (t). Acceptor radioactivity in the absence of CETP (usually <2 to 3%) is subtracted before calculation of kt values. Calculations assume steady-state conditions in which all lipid transfer is an exchange process. In order to minimize calculation errors due to mass transfer, all values were determined from assays in which the extent of radiolabel transfer was small (<15%).
Plasma lipoprotein separation.
Plasma was separated into its HDL, LDL, TRL, and lipoprotein-deficient plasma (LPDP; which primarily contains albumin and α1-glycoprotein) fractions by step-gradient ultracentrifugation, as described previously (22).
To ensure that the distribution of E5564 found in each of these fractions was a result of its association with each lipoprotein or lipoprotein-deficient fraction and not a result of the density of the formulation, the E5564 formulation was incubated in LPDP for 30 min at 37°C, and its density was determined by step-gradient ultracentrifugation, as described previously (22).
E5564 quantification and plasma and lipoprotein cholesterol analysis.
The concentration of E5564 in each lipoprotein and lipoprotein-deficient fraction was determined by counting of the radioactivity in a Beckman scintillation counter and comparison of the concentration with an external calibration curve to correct for quenching within each fraction (21). Total plasma and lipoprotein cholesterol, TG, and protein concentrations were determined by enzymatic assays purchased from Sigma Diagnostics (St. Louis, Mo.) (21).
Experimental design.
The following protocol was used to assess the influence of modified plasma and lipoprotein lipid profiles on the distribution of [14C]E5564 in plasma. [14C]E5564 at 1 μM (which is within the range of the physiological concentration observed in serum following intravenous infusion into patients [data not shown]) was incubated in plasma from seven fasted human subjects with various total cholesterol concentrations (concentration range, 103 to 294 mg/dl), total TG concentrations (concentration range, 89 to 1,332 mg/dl), and total protein concentrations (concentration range, 187 to 1,451 mg/dl) for 5, 30, 60, 180, and 360 min at 37°C. Following incubation at each time point, the plasma fractions were separated into their plasma lipoprotein and LPDP fractions by step-gradient ultracentrifugation. Each of these fractions was analyzed for its E5564 content by counting of the radioactivity and comparison of the amount with an external calibration curve for each fraction to correct for quenching. The plasma and lipoprotein cholesterol, TG, and protein concentrations were determined and correlated to the percentage of E5564 recovered in each fraction. Human plasma samples that had similar total and lipoprotein lipid profiles and very low plasma HDL levels, which are often observed in septic patients, were also used (1).
To determine which subfraction(s) of HDL binds to E5564, [14C]E5564 at 1 μM was incubated in human plasma for 30 min at 37°C. Following incubation the plasma was separated into its HDL2 and HDL3 fractions by an ultracentrifugation method developed by Groot et al. (8), and each fraction was assayed for radioactive E5564. Separation of HDL into its different subfractions was confirmed by gel electrophoresis, as described previously (10).
To further determine if the E5564 incorporated into TRL prior to incubation in plasma would redistribute to HDL and other plasma proteins, E5564 preincubated with TRL for 5 min was incubated in hypolipidemic human plasma for 5 min or 6 h at 37°C, and its distribution profile in plasma was determined.
The following investigation was done to determine if the lipoprotein distribution of E5564 was influenced by the lipoprotein surface lipid charge. Phosphatidylinositol (PI; final concentration in blood, 40 μΜ) was administered intravenously to male New Zealand White rabbits (body weight, 3 to 3.5 kg) via the marginal ear vein (17). Blood was removed 10 min after injection, and the plasma was retrieved. Radiolabeled E5564 ([3H]E5564) at 1 μΜ was incubated for 60 min at 37°C in control and PI-treated rabbit plasma. Following incubation, the plasma was separated into its lipoprotein and LPDP fractions by density gradient ultracentrifugation, and the percentage of [3H]E5564 recovered in each fraction was determined by counting the radioactivity. To determine the lipoprotein surface charge for control and PI-treated plasma, the zeta potential of each lipoprotein fraction was determined. The effect of PI administration on lipoprotein surface charge was further confirmed by gel electrophoresis (17).
To determine if E5564 influences CETP-mediated transfer of CE between HDL and LDL, increasing concentrations of E5564 (0.25 to 1.0 μΜ) were coincubated with [3H]CE-labeled HDL or LDL for 90 min at 37°C. The percentage of [3H]CE transferred between lipoproteins was determined and compared to the percentages transferred for nontreated controls and positive controls (which were treated with TP2, a monoclonal antibody directed against CETP [20]).
Statistical analysis.
The distribution of E5564 in plasma was compared between the different groups of plasma by the Mann-Whitney nonparametric test. Critical differences were assessed by Newman-Keuls post hoc tests. Correlation coefficients between the amount of E5564 recovered within the TRL, LDL, and HDL plasma fractions and the amount of cholesterol, TG, and protein within these fractions were determined by Pearson's test (see Table 2). The interday coefficient of variation (range, 5 to 8%) for the detection of E5564 within each of the lipoprotein and LPDP fractions of each patient's plasma was also determined. A difference was considered significant if the probability that chance explained the results was reduced to less than 5% (P < 0.05). All data were expressed as the means ± standard deviations.
TABLE 2.
Correlation coefficients between percentage of E5564 recovered in each lipoprotein fraction with total and lipoprotein lipid and protein concentrations for seven different patients following incubation of [14C]E5564a
| Fraction and component measured |
r (P value) for E5564 recovered in:
|
||
|---|---|---|---|
| TRL | LDL | HDL | |
| Total | |||
| TC | 0.91 (0.005) | 0.15 (NS) | −0.88 (0.01) |
| TG | 0.93 (0.003) | 0.44 (NS) | −0.98 (0.001) |
| TP | 0.28 (NS) | 0.61 (NS) | −0.01 (NS) |
| TRL | |||
| TC | 0.85 (0.02) | 0.03 (NS) | −0.84 (0.02) |
| TG | 0.91 (0.005) | 0.13 (NS) | −0.99 (0.001) |
| TP | 0.95 (0.001) | −0.03 (NS) | −0.98 (0.001) |
| LDL | |||
| TC | −0.11 (NS) | 0.85 (0.01) | 0.46 (NS) |
| TG | 0.80 (0.05) | 0.73 (NS) | −0.58 (NS) |
| TP | 0.40 (NS) | 0.97 (0.001) | −0.92 (0.005) |
| HDL | |||
| TC | 0.43 (NS) | −0.19 (NS) | −0.58 (NS) |
| TG | 0.92 (0.005) | 0.90 (0.006) | −0.97 (0.001) |
| TP | −0.25 (NS) | 0.22 (NS) | 0.08 (NS) |
E5564 at 1.0 μM was incubated in plasma from six human subjects with various lipid profiles for 30 min at 37°C. The data represent the r values between the percentage of [14C]E5564 associated with lipoproteins and plasma lipoprotein lipid and protein concentrations. TRLs include very low density lipoproteins and chylomicrons. NS, not significant; Abbreviations: TC, total cholesterol; TG, total triglyceride; TP, total protein. Statistically significant differences are represented in boldface.
RESULTS
Time course studies of plasma lipoprotein distribution of E5564.
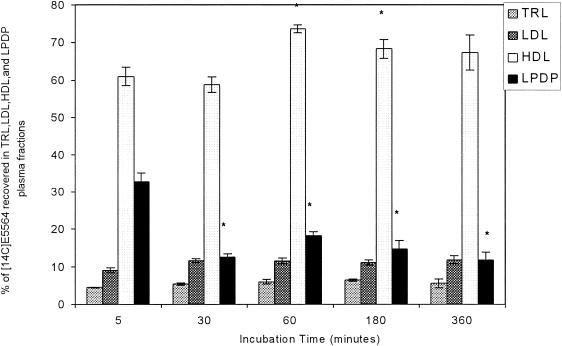
Initial studies were designed to determine if the plasma distribution profile of E5564 was time dependent. [14C]E5564 at 1 μM was incubated for 5, 30, 60, 180, and 360 min in normocholesterolemic plasma (total cholesterol concentration, 161 mg/dl); and the plasma distribution profile was determined. The majority of the drug was recovered in the HDL fraction for all incubation times (Fig. 1). Total recovery was greater than 80% (range, 80.4 to 106.9%) for all samples tested. Similar results were observed when E5564 was incubated in dyslipidemic plasma (data not shown). In both types of plasma samples, increases in the incubation time from 5 to up to 360 min did not alter the distribution profile for E5564. On the basis of these observations, the results of all studies reported in the remainder of this report were obtained by use of an incubation time of 30 min.
FIG. 1.
Distribution of [14C]E5564 (1 μM) in plasma following incubation in normolipidemic human plasma for 5, 30, 60, 180, and 360 min at 37°C. Plasma was separated into different lipoprotein and lipoprotein-deficient fractions by step-gradient ultracentrifugation. TRLs include chylomicrons and very low density lipoproteins, and the LPDP includes α1-glycoprotein and albumin. The data are presented as means ± standard deviations (n = 6). *, P < 0.05 versus 5-min incubation time.
Distribution of E5564 following incubation in plasma from human subjects with various lipid concentrations.
Initially, the distribution of [14C]E5564 at 1 μM within normolipidemic and dyslipidemic plasma from different patients following incubation for 30 min at 37°C was determined. As shown in Table 1, as the lipid content within the TRL fraction increases (hypercholesterolemic and hypertriglyceridemic groups) or as the TG content within the HDL fraction decreases (the hypoalphalipoproteinemic group), more E5564 is recovered in the TRL fraction. Overall, the total recovery was greater than 80% (range, 80.4 to 106.9%). This is acceptable, since a small percentage of the drug may stick to the surface of the centrifuge tubes (due to its hydrophobicity).
TABLE 1.
Distribution of [14C]E5564 at 1 μM in plasma from different human plasma samples following incubation for 0.5 h at 37°Ca
| Sample and sample profile | % of initial E5564 concnb
|
% Recoveryc | |||
|---|---|---|---|---|---|
| HDL fraction | TRL fraction | LDL fraction | LPDP fraction | ||
| Sample I (n = 4; total chold, 103 mg/dl; total TG, 89 mg/dl; total protein, 187 mg/dl) | 62.8 ± 6.9 | 5.5 ± 0.5 | 8.7 ± 1.1 | 7.7 ± 1.4 | 84.8 |
| Sample II (n = 4; total chol, 132 mg/dl; total TG, 140 mg/dl; total protein, 242 mg/dl) | 66.2 ± 6.2 | 3.4 ± 0.4 | 5.1 ± 0.4 | 5.6 ± 1.0 | 80.4 |
| Sample III (n = 5; total chol, 140 mg/dl; total TG, 153 mg/dl; total protein, 701 mg/dl) | 58.8 ± 2.1 | 5.4 ± 0.3 | 11.6 ± 0.5 | 12.6 ± 0.8 | 88.3 |
| Sample IV (n = 5; total chol, 180 mg/dl; total TG, 191 mg/dl; total protein, 713 mg/dl) | 61.1 ± 3.0 | 15.5 ± 0.9 | 8.2 ± 0.8 | 8.8 ± 0.9 | 93.7 |
| Sample V (n = 5; total chol, 183 mg/dl; total TG, 378 mg/dl; total protein, 636 mg/dl) | 56.5 ± 3.9 | 27.0 ± 2.7 | 14.0 ± 1.1 | 9.4 ± 0.7 | 106.9 |
| Sample VI (n = 4; total chol, 251 mg/dl; total TG, 405 mg/dl; total protein, 257 mg/dl) | 45.8 ± 3.6 | 22.6 ± 1.5 | 8.5 ± 0.5 | 4.6 ± 0.4 | 81.4 |
| Sample VII (n = 5; total chol, 294 mg/dl; total TG, 1,332 mg/dl; total protein, 1,451 mg/dl) | 9.4 ± 0.8 | 46.2 ± 5.3 | 26.5 ± 2.2 | 1.2 ± 0.3 | 83.4 |
Plasma samples were assayed for E5564 by measurement of the amount of radioactivity in each of the lipoprotein and lipoprotein-deficient plasma fractions. Lipoprotein fractions were separated by density gradient ultracentrifugation.
Data are presented as means ± standard deviations.
Percentage of the initial amount of drug incubated.
chol, cholesterol.
In the next series of studies, the correlation coefficients (r values, obtained by Pearson's test) between the amount of E5564 recovered in each lipoprotein fraction and the plasma lipoprotein lipid and protein contents from different human subjects described in Table 1 were determined. A positive statistically significant correlation between the amount of E5564 recovered in the TRL fraction and the TC, TG, and TP contents in this fraction was observed (Table 2). A positive statistically significant correlation between the amount of E5564 recovered in the LDL fraction and the TC and TP contents in this fraction was observed (Table 2). A negative statistically significant correlation between the amount of E5564 recovered in the HDL fraction and the TG content in this fraction was observed (Table 2). When E5564 was incubated in these plasma samples at a concentration range of 0.25 to 1.5 μM, plasma distribution profiles similar to those obtained with 1 μM were observed (data not shown).
Distribution of E5564 within different subclasses of HDL.
In further analyses to determine if E5564 preferentially associates with a subclass of HDL, we determined the chemical composition (dry weight) of plasma HDL2 and HDL3 and the E5564 concentrations recovered in HDL2 and HDL3 following incubation of [14C]E5564 in human plasma for 30 min at 37°C. As shown in Table 3, the majority of E5564 was recovered in HDL3, which has a greater protein content than HDL2 but lower core lipid and surface lipid contents than HDL2.
TABLE 3.
Chemical composition (by dry weight) of plasma HDL2 and HDL3 levels and E5564 concentrations recovered in HDL2 and HDL3 following incubation of 1 μM E5564 in normolipidemic human plasmaa for 30 mins at 37°C
| Fraction | % of initial E5564 incubatedb | Mr (106) | Density (g/cm3) | Percent
|
||||
|---|---|---|---|---|---|---|---|---|
| Protein | Phospholipids | TG | CE | Free cholesterol | ||||
| HDL2 | 9.3 ± 1.6 | 0.36 | 1.09 | 41 | 30 | 4.5 | 16 | 5.4 |
| HDL3 | 46.1 ± 1.1c | 0.18 | 1.15 | 55 | 23 | 4.1 | 12 | 2.9 |
Total cholesterol concentration, 135 mg/dl.
Data are presented as means ± standard deviations (n = 6 replicates) for the drug distribution work.
P < 0.05 versus HDL2.
Lipoprotein distribution of E5564 incorporated into TRL prior to incubation in human plasma.
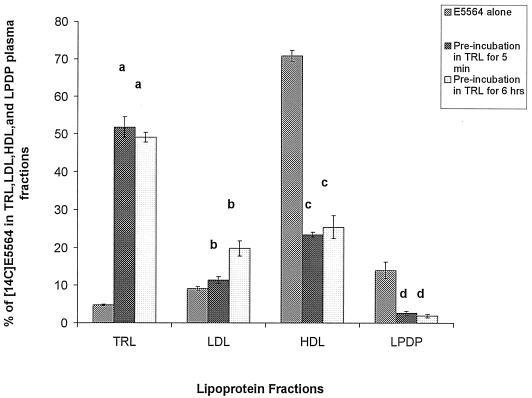
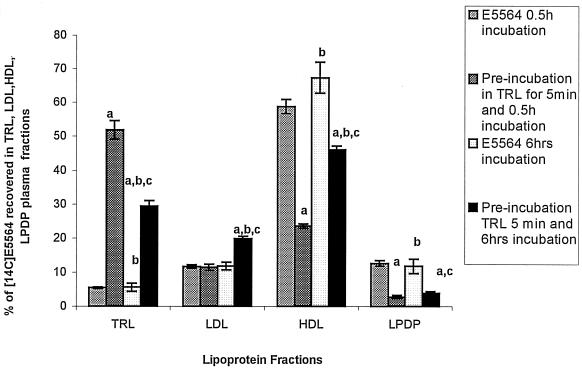
To investigate the stability of the association of E5564 with lipoproteins, we studied the possibility that E5564 would redistribute into HDL after being preequilibrated in TRL. Control incubations of [14C]E5564 (1 μΜ) in hypolipidemic plasma (total cholesterol concentration, 89 mg/dl) demonstrated 70% binding to HDL and 15% binding to LDL and very low density lipoprotein. When [14C]E5564 at 1 μM was incorporated into TRL by preincubation of E5564 in TRL for 5 or 360 min, approximately 50% was found in TRL. Addition of these samples preincubated TRL to hypolipidemic human plasma for 30 min (Fig. 2) or 360 min (Fig. 3) resulted in the binding of 22 to 48% of the E5564 to HDL, which is a 22 to 48% decrease in the amount of drug recovered in the HDL fraction, with a concomitant increase in the percentage of drug recovered in the LDL and TRL fractions compared to the amount of [14C]E5564 alone recovered.
FIG. 2.
Distribution in plasma of 1 μM [14C]E5564 incubated in saline or [14C]E5564 preincubated in TRL for 5 min or 6 h and then added to hypocholesterolemic human plasma and incubated for 0.5 h at 37°C. The plasma was separated into different lipoprotein and lipoprotein-deficient fractions by step-gradient ultracentrifugation. TRLs include chylomicrons and very low density lipoproteins, and LPDP includes α1-glycoprotein and albumin. The data are presented as means ± standard deviations (n = 6). a, P < 0.05 versus E5564 alone in TRL; b, P < 0.05 versus E5564 alone in LDL; c, P < 0.05 versus E5564 alone in HDL; d, P < 0.05 versus E5564 alone in LPDP.
FIG. 3.
Distribution in plasma of 1 μM [14C]E5564 incubated in saline or [14C]E5564 preincubated in TRL for 5 min and then added to hypocholesterolemic human plasma and incubated for 0.5 or 6 h at 37°C. The plasma was separated into different lipoprotein and lipoprotein-deficient fractions by step-gradient ultracentrifugation. TRLs include chylomicrons and very low density lipoproteins, and LPDP includes α1-glycoprotein and albumin. The data are presented as means ± standard deviations (n = 6). a, P < 0.05 versus E5564 alone for 0.5 h of incubation; b, P < 0.05 versus preincubation in TRL for 5 min and 0.5 h of incubation; c, P < 0.05 versus E5564 alone for 6 h of incubation.
Influence of lipoprotein surface charge on distribution of E5564 in plasma.
PI treatment caused the LDL and TRL fractions to migrate farther on the agarose gel, indicative of an increased negative surface charge (17; data not shown). Zeta potential analysis further showed that control LDL particles had a surface potential of −11.4 ± 1.9 mV and that PI-treated particles had a surface potential of −17.4 ± 3 mV. In addition, control TRL particles had a surface potential of −10.5 ± 1.5 mV and PI-treated particles had a surface potential of −18.3 ± 1.9 mV. No changes in the surface potential of HDL were observed. No changes in particle size, lipid and protein composition, or hydrophobic volume were observed; nor were changes in physical structure observed by transmission electron microscopy of control and PI-treated lipoproteins (17; data not shown). A greater percentage of [14C]E5564 was recovered in the PI-treated LDL and TRL fractions than in the controls (Table 4). However, no significant changes in the percentage of [14C]E5564 recovered in the HDL fractions was observed.
TABLE 4.
Lipoprotein particle surface charge as measured by zeta potential and E5564 distribution in control and PI-treated rabbit plasmaa
| Plasma sample | Zeta potential (mV)
|
E5564 distribution (%)b
|
|||||
|---|---|---|---|---|---|---|---|
| HDL | LDL | TRL | HDL | LDL | TRL | LPDP | |
| Control | −18.9 ± 1.4 | −11.4 ± 1.9 | −10.5 ± 1.5 | 85.5 ± 1.3 | 3.2 ± 0.1 | 1.2 ± 0.1 | 4.0 ± 1.1 |
| PI treated | −18.1 ± 2.8 | −17.4 ± 3.0c | −18.3 ± 1.9c | 81.3 ± 2.7 | 6.9 ± 0.4c | 1.9 ± 0.1c | 6.7 ± 0.9c |
Data are reported as means ± standard deviations (n = 3). TRL contains chylomicrons and very low density lipoproteins. LPDP contains albumin and α1-glycoprotein.
Percentage of original E5564 (1 μM) incubated in plasma.
P < 0.05 versus control plasma.
Influence of E5564 on CETP-mediated transfer of CE between lipoproteins.
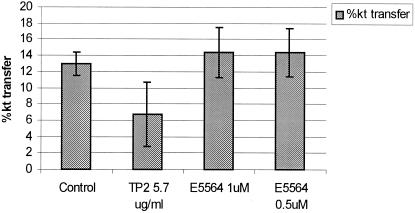
E5564 at concentrations up to 1 μM did not inhibit the CETP-mediated transfer of CE between HDL and LDL (Fig. 4). As a control, TP2, a monoclonal antibody against CETP, significantly decreased the level of CETP-mediated transfer activity, as reported previously (20).
FIG. 4.
Percent transfer of CE from LDL to HDL in the presence or absence of a monoclonal antibody (TP2) directed against CETP or increasing concentrations of E5564 (0.5 and 1.0 μM) following incubation of radiolabeled CE-enriched LDL with cold HDL (with 20 μg of lipoprotein phosphatidylcholine) for 90 min at 37°C in T150 buffer supplemented with CETP (1.0 μg of protein/ml) or delipidated human plasma with 1.0 μg of protein/ml of CETP. The data are expressed as means ± standard deviations (n = 6). For the data for TP2, P < 0.05 versus the control.
DISCUSSION
Patients with sepsis or other diseases often exhibit alterations in plasma lipoprotein lipid and protein concentrations (3, 6, 9, 14, 18, 19). The purpose of the studies described here was to determine if alterations in plasma lipid and protein concentrations could modify the plasma lipoprotein distribution of E5564. We observed that in normolipidemic plasma, the majority of E5564 (>74.8%) was recovered in the plasma lipoprotein fraction, primarily in the HDL fraction (>56.5%; Table 1). We further observed that increases in TRL lipid (cholesterol and TG) and protein concentrations significantly increased the percentage of E5564 recovered in this non-HDL fraction while decreasing the percentage of drug recovered in the HDL fraction (Table 2).
Previous studies with the immunosuppressant cyclosporine (CSA) have suggested that alterations in plasma lipid concentrations modify the pharmacological behavior of CSA. Lemaire and coworkers (11, 12) have observed enhanced antiproliferative effects of CSA when the drug was bound to LDL that were not evident when the drug was bound to either very low density lipoprotein or HDL. Gardier and coworkers (7) observed that heart transplant patients with high total plasma cholesterol levels demonstrated an increased association of CSA with plasma LDL and an increased CSA-induced renal toxicity compared to the results for normolipidemic controls. Arnadottir and coworkers (2) observed elevations in CSA-induced renal toxicity in kidney transplant patients who exhibited increases in plasma cholesterol concentrations.
Studies by Rose and coworkers (16) have supported the importance of plasma lipoprotein binding in influencing the long-term effectiveness of E5531. In their studies they observed that E5531 was slowly inactivated when it was bound to HDL, while little or no loss of activity occurred when it was associated with LDL, as determined by in vitro assays (16). Preliminary studies with E5564 have yielded similar results (data not shown). These studies provide compelling evidence suggesting that the plasma lipoprotein lipid level could affect the distributions and pharmacological activities of E5531 and E5564 in plasma.
In the present study, we observed that, except for incubation in the most dyslipidemic human plasma samples, the majority of E5564 added was recovered in the HDL fraction (Table 1). By cholesterol content, LDL is more abundant than HDL at LDL:HDL ratios of 4:1 to 6:1 (23), indicating that some mechanism besides random probability may dictate E5564's preferential association with HDL rather than LDL. This is supported by the fact that nearly 50% of HDL by weight is composed of protein (10) and by our observations that a greater percentage of E5564 was recovered in the HDL3 subfraction (which contains more protein) than in the HDL2, TRL, and LDL fractions (which contain less protein) (Table 3).
We have further observed that the use of various plasma lipoprotein levels and specific increases in LDL and TRL lipid and protein levels resulted in the recovery of increasing percentages of E5564 in these fractions (Table 2). These findings suggest that the E5564 distribution in lipoprotein fractions may be partially dictated by LDL and TRL lipid mass and lipoprotein particle number or surface area. It further suggests that the distribution of E5564 from one lipoprotein class (HDL) to another (LDL or TRL) could be influenced by different disease states (9, 18, 19) and adjunct therapies such as intralipid infusion (24), which result in altered plasma lipoprotein concentrations and compositions.
The results from these studies suggest that increases in TG levels cause more of the drug to be recovered within the TRL fraction and cause the drug to be less associated with the HDL fraction. On the basis of these results, experiments were designed to determine if preincubation of E5564 with TRL would result in a stable association of E5564 with non-HDL lipoproteins. When E5564 was preincubated in purified TRL, the majority of the drug was recovered in the TRL fraction and less was recovered in the HDL fraction after incubation for 0.5 h compared with the results obtained for the control incubation (Fig. 2 and 3).
Taken together these data suggest that the distribution of E5564 into different lipoproteins may be influenced by the solubility of the drug in the lipid components of the TRL and/or LDL fraction, the affinity of the drug for the protein component of HDL, and the surface area and the number of lipoprotein particles available. Although the drug appears to have a high level of solubility in core apolar lipids (CE and TG), the hydrophobic volume of HDL is quite small, and thus, the drug cannot reside in the apolar core of the HDL fraction. When E5564 associates with TRL and/or LDL, it predominantly interacts with the apolar lipid core of the lipoprotein fractions. This conclusion is supported by the observations that the drug appears to have a high level of solubility in core apolar lipids (data not shown) and that the hydrophobic core volumes of these lipoproteins are larger than that the hydrophobic core volume of HDL (10) and also correlates with the percentage of E5564 recovered in these fractions. Furthermore, it does not appear that changes in LDL and TRL surface charges influence the distribution of E5564 in the HDL fraction (Table 4). This finding provides additional evidence that the drug does not reside in the lipid coat of lipoproteins but, rather, either resides in the apolar lipid core or is bound to the protein component of lipoproteins. In addition, we observed that E5564 does not influence the CETP-mediated transfer activity of CE between lipoproteins (Fig. 4). These findings further support the hypothesis that E5564 primarily associates with the protein component of HDL. Due to this interaction, the drug is unavailable to interact with CETP. Further studies to determine the pharmacological relevance of these findings are warranted.
In summary, the findings of the present study suggest that the majority of E5564 is bound to HDL and that binding to lipoproteins is fast (within 5 min), with no measurable redistribution of E5564 between different lipoprotein fractions. Furthermore, it appears that increases in plasma TRL lipid and protein levels significantly increase the level of association of E5564 with TRL and decrease the level of association of E5564 with HDL. In addition, preincubation of E5564 in TRL prior to incubation in plasma significantly decreased the percentage of drug recovered in the HDL fraction. The information obtained from these studies could be used to help us identify the possible components of lipoproteins which influence the interaction of E5564 with specific lipoprotein particles.
Acknowledgments
Funding for this work was provided by Eisai Medical Research Inc. and the Canadian Institutes of Health Research (MOP 14484) to K.M.W.
REFERENCES
- 1.Alvares, C., and A. Ramos. 1986. Lipids, lipoproteins, and apoproteins in serum during infection. Clin. Chem. 32:142-145. [PubMed] [Google Scholar]
- 2.Arnadottir M., H. Thysell, and P. Nilsson-Ehle. 1991. Lipoprotein levels and post-heparin lipase activities in kidney transplant recipients: ciclosporin-versus nonciclosporin-treated patients. Am. J. Kidney Dis. 17:700-717. [DOI] [PubMed] [Google Scholar]
- 3.Bone, R. C. 1991. Gram negative sepsis: background, clinical features, and intervention. Chest 100:802-808. [DOI] [PubMed] [Google Scholar]
- 4.Burstein, M., H. R. Scholnick, and R. Morfin. 1970. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J. Lipid Res. 11:583-595. [PubMed] [Google Scholar]
- 5.Christ, W. J., O. Asano, L. C. Robidoux, et al. 1995. E5531, a pure endotoxin antagonist of high potency. Science 268:80-83. [DOI] [PubMed] [Google Scholar]
- 6.Feingold, K. R., R. M. Krauss, M. Pang, et al. 1993. The hypertriglyceridemia of acquired immunodeficiency syndrome is associated with an increased prevalence of low-density lipoprotein subclass pattern. J. Clin. Endocrinol. Metab. 76:1423-1431. [DOI] [PubMed] [Google Scholar]
- 7.Gardier, A. M., D. Mathe, X. Guedeney, J. Barre, et al. 1993. Effects of plasma lipid levels on blood distribution and pharmacokinetics of cyclosporin A. Ther. Drug Monit. 15:274-280. [DOI] [PubMed] [Google Scholar]
- 8.Groot, P. H. E., L. M. Scheek, L. Havekes, et al. 1982. A one-step separation of human high-density lipoproteins 2 and 3 by rate-zonal density gradient ultracentrifugation in a swinging bucket rotor. J. Lipid Res. 23:1342-1353. [PubMed] [Google Scholar]
- 9.Grunfeld, C., M. Pang, W. Doerrler, et al. 1992. Lipids, lipoproteins, triglyceride clearance and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J. Clin. Endocrinol. Metab. 74:2045-2051. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy, A. L., and K. M. Wasan. 1999. Preferential distribution of amphotericin B lipid complex into human HDL3 is a consequence of high density lipoprotein coat lipid content. J. Pharm. Sci. 88:1149-1155. [DOI] [PubMed] [Google Scholar]
- 11.Lemaire, M., and W. M. Pardridge. 1988. Influence of blood components on the tissue uptake indices of cyclosporin in rats. J. Pharmacol. Exp. Ther. 244:740-743. [PubMed] [Google Scholar]
- 12.Lemaire, M., and J. P. Tillement. 1982. Role of lipoproteins and erythrocytes in the in vitro binding and distribution of cyclosporin A in the blood. J. Pharm. Pharmacol. 34:715-718. [DOI] [PubMed] [Google Scholar]
- 13.Morton, R. E., and D. B. Zilversmit. 1982. Purification and characterization of lipid transfer protein(s) from human lipoprotein-deficient plasma. J. Lipid Res. 23:1058-1067. [PubMed] [Google Scholar]
- 14.Parrillo, J. E. 1993. Pathogenetic mechanisms of septic shock. N. Engl. J. Med. 328:1471-1477. [DOI] [PubMed] [Google Scholar]
- 15.Pattnaik, N. M., and D. B. Zilversmit. 1979. Interaction of cholesteryl ester exchange protein with human plasma lipoproteins and phospholipid vesicles. J. Biol. Chem. 254:2782-2786. [PubMed] [Google Scholar]
- 16.Rose, J., M. Mullarkey, W. J. Christ, L. D. Hawkins, M. Lynn, Y. Kishi, K. M. Wasan, K. D. Peteherych, and D. P. Rossignol. 2000. Consequences of interaction of a lipophilic endotoxin antagonist with plasma lipoproteins. Antimicrob. Agents Chemother. 44:504-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stamler, C. J., D. Breznan, T. A. Neville, F. J. Viau, E. Camlioglu, and D. L. Sparks. 2000. Phosphatidylinositol promotes cholesterol transport in vivo. J. Lipid Res. 41:1214-1221. [PubMed] [Google Scholar]
- 18.Umeki, S. 1993. Decreases in serum cholesterol levels in advanced lung cancer. Respiration 60:178-181. [DOI] [PubMed] [Google Scholar]
- 19.Vitols, S., G. Gahrton, M. Bjorkholm, and C. Peterson. 1985. Hypocholesterolemia in malignancy due to elevated low-density-lipoprotein-receptor activity in tumor cells: evidence from studies in patients with leukemia. Lancet iv:1150-1154. [DOI] [PubMed]
- 20.Wasan, K. M., M. Ramaswamy, W. Wong, and P. H. Pritchard. 1998. Lipid transfer protein I facilitated transfer of cyclosporine from low- to high-density lipoproteins is only partially dependent on its cholesteryl ester transfer activity. J. Pharmacol. Exp. Ther. 284:599-605. [PubMed] [Google Scholar]
- 21.Wasan, K. M., F. W. Strobel, S. C. Parrott, M. Lynn, W. J. Christ, L. D. Hawkins, and D. P. Rossignol. 1999. Lipoprotein distribution of a novel endotoxin antagonist, E5531, in plasma from human subjects with various lipid levels. Antimicrob. Agents Chemother. 43:2562-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wasan, K. M., S. M. Cassidy, M. Ramaswamy, A. Kennedy, F. W. Strobel, S. P. Ng, and T. P. Lee. 1999. A comparison of step-gradient and sequential density ultracentrifugation and the use of lipoprotein deficient plasma controls in determining the plasma lipoprotein distribution of hydrophobic compounds. Pharm. Res. 16:165-169. [DOI] [PubMed] [Google Scholar]
- 23.Wasan, K. M., and G. Lopez-Berestein. 1995. Targeted liposomes in fungi: modifying the therapeutic index of amphotericin B by its incorporation into negatively charged liposomes. J. Liposome Res. 5:883-903. [Google Scholar]
- 24.Wasan, K. M., V. B. Grossie, Jr., and G. Lopez-Berestein. 1994. Concentrations in serum and tissue distribution of free and liposomal amphotericin B in rats on continuous intralipid infusion. Antimicrob. Agents Chemother. 38:2224-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]