Abstract
Staphylococcal infections associated with catheter and prosthetic implants are difficult to eradicate and often lead to chronic infections. Development of novel antibacterial therapies requires simple, reliable, and relevant models for infection. Using bioluminescent Staphylococcus aureus, we have adapted the existing foreign-body and deep-wound mouse models of staphylococcal infection to allow real-time monitoring of the bacterial colonization of catheters or tissues. This approach also enables kinetic measurements of bacterial growth and clearance in each infected animal. Persistence of infection was observed throughout the course of the study until termination of the experiment at day 16 in a deep-wound model and day 21 in the foreign-body model, providing sufficient time to test the effects of antibacterial compounds. The usefulness of both animal models was assessed by using linezolid as a test compound and comparing bioluminescent measurements to bacterial counts. In the foreign-body model, a three-dose antibiotic regimen (2, 5, and 24 h after infection) resulted in a decrease in both luminescence and bacterial counts recovered from the implant compared to those of the mock-treated infected mice. In addition, linezolid treatment prevented the formation of subcutaneous abscesses, although it did not completely resolve the infection. In the thigh model, the same treatment regimen resulted in complete resolution of the luminescent signal, which correlated with clearance of the bacteria from the thighs.
Staphylococcus aureus and coagulase-negative staphylococci (CoNS) are the leading causes of nosocomial infections in the United States, accounting for approximately 50% of all cases (27). Many of these infections are life threatening, resulting in diseases such as pneumonia, septicemia, and endocarditis (17). An additional confounding problem is an increasing incidence of antibiotic resistance. Approximately 50% of the hospital-acquired strains of S. aureus and 90% of CoNS isolates are resistant to methicillin (7). Recently, S. aureus strains which are resistant to vancomycin have been reported (24), making this problem even more serious. New approaches are needed for both the prevention and treatment of these infections, and robust, reproducible animal models are required to evaluate the approaches. Many of the published models of staphylococcal infection suffer from shortcomings, with experimental end points requiring determination of the degree of swelling, (12), abscess formation (26), or number of recovered CFU (19). CFU determination is an indirect and time-consuming method that involves laborious procedures, such as harvesting and homogenization of tissues, serial plating of infectious agents, and colony counting. Furthermore, because animals are sacrificed at each time point, large numbers of animals must be used, and the course of the disease cannot be directly monitored over time (4, 23). A potential solution to these drawbacks is to use biophotonic imaging, as first described by Contag et al. (8) and later by Burns et al. (6) for monitoring gram-negative bacterial infections. This approach has also been used to monitor S. aureus strains by engineering them to express the luciferase operon (lux) from Photorhabdus luminescens (6, 21). Bacteria that contain this cassette as stable transformants are capable of producing both the luciferase enzyme and its substrate, thereby emitting a bioluminescent signal when the bacteria are metabolically active. The intensity of the light emitted by the bacteria is sufficient for the signal to pass through the tissue of a live mouse or other laboratory animal. This signal can then be quantified using a highly sensitive charge-coupled device (CCD) digital camera and image analysis software (14, 21). Such an imaging system has been used successfully to monitor short-term bacterial infection with Escherichia coli and S. aureus in the thigh model in real time (14, 21). However, in those experiments, the luciferase cassette was maintained on a plasmid in the bacteria rather than stably integrated into the genome, permitting only short-term (24-h) monitoring of the bacterial infection.
Our aim was to determine whether two staphylococcal murine animal models, the foreign-body model (22), in which biofilm formation by the inoculum on the implanted catheter results in a persistent infection (9, 10), and the deep-wound model (11), where bacteria persist by adhering to cellular components (13), could be adapted to allow disease progression to be monitored by biophotonic imaging of a bioluminescent S. aureus strain having a chromosomally integrated copy of a lux operon. We asked whether this approach could permit continuous in vivo real-time evaluation of bacterial colonization and pathogenesis for an extended period after infection. The long-term monitoring of bacterial infection is necessary to evaluate the efficacy of antibiotics and/or potential vaccine candidates. Here we report the adaptation of both models for use with a bioluminescent strain of S. aureus. We have used the antibiotic linezolid (Zyvox), which has been demonstrated to have antistaphylococcal activity (1-3, 16, 20), as a model compound to demonstrate the use of bioluminescent bacteria in animal models in an antibacterial screening program.
MATERIALS AND METHODS
Reagents.
Bioluminescent S. aureus was generated by transforming strain 8325-4 with a modified P. luminescens lux operon using the gram-positive lux transposon plasmid pAUL-ATn4001 luxABCDE Kmr, which was introduced into the cells by electroporation as previously described (14, 15). Transformants were grown overnight in Trypticase soy broth (TSB) containing erythromycin (5 μg/ml) and then plated onto TSB medium containing kanamycin (200 μg/ml) to select for those clones where the Tn4001 luxABCDE Kmr cassette had transposed and inserted downstream of a promoter. Highly bioluminescent colonies were selected using an IVIS Imaging System (Xenogen Corporation, Alameda, Calif.). One clone, designated S. aureus Xen8.1, was selected and further characterized. Staphylococcus epidermidis strain RP62A was obtained from the American Type Culture Collection (Manassas, Va.) as ATCC 35984. Both bacterial strains were grown at 37°C to mid-exponential phase in TSB (Becton-Dickinson, Cockeysville, Md.), resuspended in phosphate-buffered saline (PBS) containing 10% glycerol (vol/vol), aliquoted, and stored at −80°C. Those inocula were thawed, diluted appropriately, and used for infection. Linezolid (Zyvox) at a concentration of 2 mg/ml was purchased from Pharmacia & Upjohn, Kalamazoo, Mich.
Mice.
Ten- to 12-week-old female BALB/c mice were obtained from Taconic Laboratories (Germantown, N.Y.). All animals were housed in the animal facility on site, and all experimental procedures adhered to a protocol approved by the Institutional Animal Care and Use Committee.
Measurement of bioluminescence.
After infection with bioluminescent S. aureus Xen8.1, the mice were anesthetized (ketamine [100 mg/ml] and xylazine [20 mg/ml] mixed in a 4:1 ratio [vol/vol] before use) and imaged as previously described (14). Briefly, photons emitted from the metabolically active S. aureus were collected during a 5-min exposure using the IVIS Imaging System and Living Image software (Xenogen Corporation). Bioluminescent images were displayed using a pseudocolor scale (blue representing the least-intense light and red representing the most-intense light) that was overlaid on a gray-scale image to generate a two-dimensional picture of the distribution of bioluminescent bacteria in the animal. The acquired image data were saved as two-dimensional arrays containing values corresponding to the number of photons contained within each pixel. The image data were subsequently transferred to a Power Macintosh G4 and analyzed, and pictures were created using Aldus SuperPaint 3.0 (see Fig. 1 and 4). To account for the background luminescence, one uninfected mouse was imaged along with the infected animals and was positioned horizontally above the infected mice. The photon emissions from a region of interest with a 2.1-cm diameter around the infected catheters or thighs were quantified using the Living Image software package (Xenogen Corporation), and the data are presented as relative light units contained within each region.
FIG. 1.
Correlation between bacterial infection and bioluminescence in the mouse foreign-body model. (A) Mice implanted with catheters were infected with 108, 107, and 106 CFU of S. aureus Xen8.1 and were imaged immediately after infection (day 0) using the IVIS Imaging System. Pictures of 5 of 10 imaged mice are presented. (B) Correlation between infectious dose and bacterial luminescence at day 0. (C) Correlation between bioluminescent signal detected and the amount of bacteria recovered from the catheters of mice at day 3 after infection with S. aureus Xen8.1. Bioluminescence (black circles) (in relative light units [RLU]) of individual mice (n = 4 to 8) is shown in panels B and C. Geometric mean values (black bars) are shown in panel B.
FIG. 4.
Correlation between S. aureus Xen8.1 infection dose and the level of bioluminescence in the deep-wound infection model. (A) Mice implanted with catheters were infected with 1 × 108, 5 × 107, or 1 × 107 CFU of S. aureus Xen8.1 and were imaged immediately after infection (day 0) using the IVIS Imaging System. Pictures of 5 of 10 imaged mice are presented. (B) Correlation between infectious dose and bacterial luminescence at day 0. (C) Correlation between bioluminescent signal detected and the amount of bacteria recovered from the thighs of mice at day 3 after infection with S. aureus Xen8.1. Bioluminescence (black circles) (in relative light units [RLU]) of individual mice (n = 4 to 8) is shown in panels B and C. Geometric mean values (black bars) are shown in panel B.
Foreign-body model.
The foreign-body model described by Rupp et al. (22) was used with the following modifications. The back of the neck or the right flank of the anesthetized mouse was shaved, treated with depilatory cream (Nair; Carter Products, New York, N.Y.), and cleansed with repeated wiping of 70% ethanol and povidone-iodine. Using sterile procedures, a small incision was made in the prepared area of skin, and a single 1-cm-long segment of sterile 14-gauge Teflon intravenous catheter (QuickCath; Baxter, Deerfield, Ill.) was implanted into the subcutaneous (s.c.) space of each mouse. The incision was closed with tissue adhesive (Vetbond; 3M, St. Paul, Minn.). Twenty-four hours later (day 0), the mice were injected with 100 μl of PBS containing different amounts of S. epidermidis RP62A or S. aureus Xen8.1 (into the catheter bed) (range, 5 × 108 to 1 × 106 CFU). Mice were observed daily for s.c. abscess formation and were photographed at day 3 after infection. In addition, animals infected with S. aureus Xen8.1 were imaged immediately after infection and then every other day as described above.
To determine the amount of bacteria that had adhered to the catheters, mice were sacrificed at various time points, the catheters were removed aseptically and placed in 1-ml portions of sterile PBS, and the catheters in PBS were vortexed for 1 min at high speed in a Vortex Genie (Scientific Industries, Bohemia, N.Y.) to resuspend the adherent bacteria. The number of viable bacteria was determined by plating serially diluted washings on Trypticase soy agar (TSA) plates (Becton-Dickinson). When S. aureus Xen8.1 was used for infection, the vortexed catheters were incubated overnight at 37°C on TSA plates and imaged to determine whether luminescent bacteria were still present. The sterility of this technique was monitored by adding an additional group of two or three mice per experiment. These mice were mock infected with PBS, and the catheters were processed as described above.
Deep-wound model.
Groups of mice were injected in the posterior quadriceps muscle of the thigh with 100 μl of PBS containing 1 × 108, 5 × 107, or 1 × 107 CFU of S. aureus Xen8.1. The bacterial load at the wound site was monitored using the IVIS Imaging System, as described above. Mice infected with 108 CFU of Xen8.1 and treated with linezolid, as described below, were imaged at days 0, 1, 3, 6, and 16. At day 16, all the mice were sacrificed, the thighs were removed and placed in 5-ml portions of PBS containing 10% glycerol for temporary storage at 70°C or for same-day processing. Usually, plating for bacterial counts was done at a later date. On the day of plating, the tubes were thawed in cold water, and thighs were ground with a Polytron homogenizer (Brinkmann Instruments) for ∼5 to 10 s each and placed on ice to await further dilution. Tenfold serial dilutions of each sample were made in tubes containing 0.9 ml of cold TSB prior to plating 0.1 ml from selected dilutions onto staphylococcus-selective mannitol salt agar plates in duplicate. Plates were incubated for 2 days at 35°C to determine the number of CFU remaining per thigh. The geometric mean of the CFU remaining and the log10 change in CFU were also determined for each group. The colonies were counted to quantify the bacterial load, and the plates were imaged to confirm bioluminescence of the recovered bacteria.
Antibiotic treatment.
In the foreign-body model, mice infected with 108 CFU of S. aureus Xen8.1 were treated with 20 mg of linezolid per kg of body weight orally (p.o.) at 2, 5, and 24 h after infection. A second group of infected mice was given the same regimen of linezolid s.c. at least 2 cm distal to the catheter site. An infected control group received PBS without linezolid p.o. CoNS infection was used only as a control, and luminescent S. aureus Xen8.1 was the only model to be treated with linezolid. To investigate the effect of linezolid during the first 24 h after infection, a group of 24 catheter-implanted BALB/c mice were injected (in the catheter bed) with 100 μl of PBS containing 108 CFU of Xen8.1. The mice were divided in two groups and were given either linezolid (20 mg/kg) or PBS p.o. at 2 and 5 h. At 0, 8, 12, and 20 h after infection, three mice per group were imaged and sacrificed. Catheters were removed, and the bacteria recovered from catheters were quantified.
In the deep-wound model, BALB/c mice were injected in the posterior quadriceps muscle of the thigh with 108 CFU of S. aureus Xen8.1 and subsequently randomly assigned to a group (four groups total). The first group was given 20 mg of linezolid per kg p.o. at 2 h following infection. The second group was given the same treatment twice at 2 and 5 h, and the third group was treated three times at 2, 5, and 24 h. PBS without antibiotic was administered to the fourth group of infected mice as a negative control. To investigate the effect of linezolid in the thigh model during the first 24 h after infection, a group of 24 mice were infected with 100 μl of PBS containing 108 CFU of Xen8.1. The mice were divided in two groups and were given either linezolid (20 mg/kg) or PBS p.o. at 2 and 5 h. At 0, 4, 8, and 12 h after infection, three mice per group were imaged and sacrificed, and bacteria recovered from the infected thighs were quantified.
Statistical analysis.
A two-tailed t test was used to calculate the P values. Geometric mean titers (GMT) or geometric means were determined as described previously (25).
RESULTS
In vivo detection of bioluminescent S. aureus Xen8.1 colonization of catheters in the mouse foreign-body model.
The 50% lethal doses for S. aureus Xen8.1 and its parental strain 8325-4 were shown to be comparable in a sepsis model (bacterial administered via the intraperitoneal route), suggesting that integration of the lux cassette into the bacterial chromosome had no significant effect on bacterial pathogenicity or survival in mice (data not shown).
The foreign-body mouse model was designed to noninvasively measure bacterial colonization on catheters in vivo after infection with bioluminescent S. aureus Xen8.1. To determine the optimal dose for infection, mice implanted with catheters were infected with different doses of Xen8.1, and the adherence of bacteria to the catheter and their long-term persistence were monitored in vivo by monitoring the bioluminescence signal. The detected luminescence signal from the infected animals was proportional to the amount of injected bacteria (Fig. 1). As a negative control (accounting for the background luminescence), one uninfected mouse was imaged along with the infected animals (Fig. 1A, horizontal mouse). The lower limit of detection in this model at day 0 (Fig. 1B) and at day 3 after (Fig. 1C) was determined to be 1 × 105 to 5 × 105 CFU. The luminescence in mice infected with 108 CFU persisted in all animals until day 21 following infection, when the experiment was terminated (Fig. 2A). In mice infected with lower amounts of bacteria (1 × 107 or 1 × 106 CFU), the luminescence signal dropped below background levels between days 5 and 7 in 40 and 90% of the mice, respectively (data not shown), but reappeared in some mice at later time points. Therefore, 108 CFU/mouse was chosen as the optimal challenge dose for evaluating antibacterial strategies because all animals were luminescent throughout the experiment.
FIG. 2.
In vivo detection of persistent S. aureus Xen8.1 infection in the foreign-body (A) and the deep-thigh-wound (B) mouse models. Bioluminescence (in relative light units [RLU]) of individual mice (n = 10) is shown on the y axes. Geometric mean values (black bars) are shown. Uninf., uninfected.
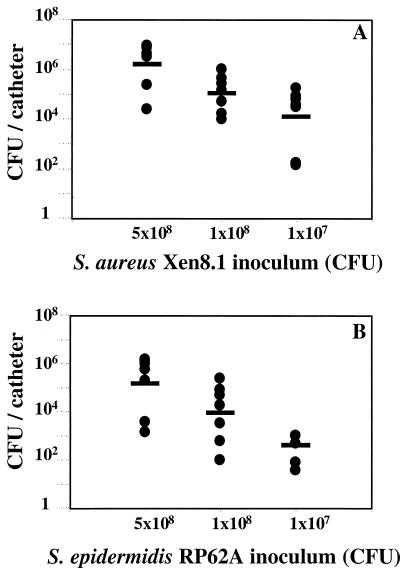
To determine whether the bioluminescent S. aureus Xen8.1 bacteria actually colonized the catheters, bacteria recovered from catheters were quantified by conventional CFU determination. For a control, we also included S. epidermidis RP62A, a strain that is known to successfully colonize implanted catheters and form a biofilm (22, 23). With both strains, the optimal challenge dose was determined to be the amount of bacteria needed to achieve reproducible infection with more than 70% of the infected catheters colonized at day 7. No colonies were recovered from catheters of mock-infected mice, indicating the probable sterility of our technique. The amount of S. aureus Xen8.1 bacteria recovered from the catheters at day 7 postinfection was proportional to the infectious dose (Fig. 3A). Bacteria were recovered from 88, 100, and 70% of the catheters infected with 5 × 108, 1 × 108, and 1 × 107 CFU of S. aureus, respectively (Fig. 3A). Similarly, bacteria were recovered from 80, 60, and 50% of the catheters infected with 5 × 108, 1 × 108, and 1 × 107 CFU of S. epidermidis, respectively (Fig. 3B). The optimal doses for S. aureus and S. epidermidis were determined to be 1 × 108 and 5 × 108, respectively (Fig. 3B). Our data demonstrate that S. aureus Xen8.1, like S. epidermidis, can be recovered from the inoculated catheters, indicating that it can effectively adhere to an implanted Teflon device (Fig. 3).
FIG. 3.
Recovery of bacteria from infected catheters. (A) Mice with implanted catheters were infected with 5 × 108, 1 × 108, or 1 × 107 CFU of S. aureus Xen8.1. At day 7, the mice were sacrificed and bacteria were recovered from 8 of 9, 8 of 8, and 7 of 10 of the catheters infected with 5 × 108, 1 × 108, and 1 × 107 CFU of S. aureus Xen8.1, respectively. (B) Mice with implanted catheters were infected with different doses of S. epidermidis RP62A. At day 7, the mice were sacrificed, and bacteria was recovered from 8 of 10, 7 of 10, and 5 of 10 of the catheters infected with 5 × 108, 1 × 108, and 1 × 107 CFU of S. epidermidis RP62A, respectively. The CFU of the infected catheters (black circles) and the GMT (black dashes) are shown. Only the infected mice were included in the GMT calculations.
Formation of s.c. abscesses.
We observed that similar to S. epidermidis (22, 23), S. aureus Xen8.1 infections induced the formation of s.c. abscesses (data not shown). The abscesses appeared at day 3 and resolved by day 6. In mock-infected animals, the surgical site healed well, and there were no signs of inflammation, thus demonstrating that the lesions were induced by the S. aureus infection rather than surgical injury. Mice infected with lower doses of bacteria (≤5 × 107CFU) did not develop abscesses (data not shown).
Establishment of a deep-wound model of infection with S. aureus Xen8.1.
In contrast to the foreign-body model where the bacteria are injected underneath the skin, in the deep-wound infection model, the bacteria are inoculated deep into the muscle. Since the luminescent signal is emitted through additional tissue layers, experiments were conducted to determine the lower limit of detection. We injected the thighs of mice with 100-μl portions of PBS containing decreasing amounts of S. aureus Xen8.1 (1 × 108, 5 × 107, 1 × 107, 1 × 106, and 1 × 105 CFU/thigh) and immediately imaged them. All of the mice infected with 1 × 108, 5 × 107, 1 × 107, and 1 × 106 CFU had detectable luminescence, whereas no luminescence was detected from the thighs of mice injected with 105 CFU or less (Fig. 4B). At day 3 after infection, the mice were imaged and then sacrificed. Comparison of CFU recovered from the thigh suspensions with the bioluminescence detected demonstrated that our limit of detection for that model was 1 × 106 to 2 × 106 CFU (Fig. 4B).
The optimal bacterial inoculum was determined to be 108 CFU (Fig. 3B and 4A). Higher infectious doses induced strong inflammation and necrosis (data not shown), whereas lower doses were below the level of luminescence detection by day 7. In mice infected with 108 CFU, a decrease and subsequent increase of luminescence was observed at day 1 and day 3, respectively. After day 7, the luminescent signal slowly decreased over time but was still above background at day 16 when the experiment was terminated (Fig. 2B).
Effect of the antibacterial agent linezolid on S. aureus in the foreign-body model.
To evaluate the use of luminescent bacteria in the foreign-body mouse model, we tested the effect of linezolid as a model antibacterial compound. Mice were infected with S. aureus Xen8.1 and subsequently given three doses of linezolid or PBS (as a control) either p.o. or s.c. Bacterial survival was monitored over time by imaging. Immediately after infection (day 0), comparable luminescence signals were detected in all groups of mice, demonstrating that they were successfully infected (Fig. 5). At day 1, statistically significant differences between the values of the untreated and linezolid-treated mice were first observed (Fig. 5). The signals from the treated groups continued to be lower at days 2 and 4. However, by day 7, the luminescence of all mice decreased, and the values in the treated and untreated groups were not statistically different (Fig. 5). In contrast to the untreated mice which all developed s.c. abscesses and had high bacterial counts recovered from the catheters, the surgical sites of the treated mice were similar to those of the mock-infected mice (data not shown), with lower bacterial counts recovered from their catheters. At day 4 after infection, the GMT of the recovered CFU from all mice in the treated groups and the untreated group were determined to be 9.10 × 102 (range, 0 to 1.9 × 104) and 2.02 × 104 (range, 1 × 102 to 1.9 × 106), respectively. Thus, in this model, while treatment with three doses of linezold prevented abscess formation, reduced luminescence signal, and bacterial colonization of the catheters, it did not result in complete resolution of the infection.
FIG. 5.
Treatment with linezolid reduced the luminescence of mice in the foreign-body model of S. aureus Xen8.1 infection. BALB/c mice were implanted with catheters and infected with 108 CFU of S. aureus Xen8.1 after 24 h. Ten infected mice were given 20 mg of linezolid per kg by oral gavage at 2, 5, and 24 h following infection. Another group of 10 infected mice was given the same regimen of linezolid but by s.c. injection. Bacterial replication was monitored over time by imaging the mice at days 0, 1, 2, 4, and 7. The data are presented as geometric means of the bioluminescence (in relative light units [RLU]) for the group of 10 mice. Statistically significant differences between the values for the untreated and treated mice were observed at day 1, day 2 (P < 0.01), and day 4 (P < 0.05). The P values for untreated and treated mice at different times after infection are as follows: for day 0, P = 0.8795 for oral treatment and P = 0.1295 for s.c. treatment; for day 1, P = 0.0099 for oral treatment and P = 0.00689 for s.c. treatment; for day 2, P = 0.0001 for oral treatment and P = 0.00007 for s.c. treatment; for day 4, P = 0.0446 for oral treatment and P = 0.03785 for s.c. treatment; for day 7, P = 0.5677 for oral treatment and P = 0.4515 for s.c. treatment. The results from one experiment are shown. Two experiments were performed with similar results. Uninf., uninfected.
To investigate the effect of linezolid during the first 24 h after infection, mice were infected with S. aureus Xen8.1 and randomly assigned to one of two groups. One group was treated with linezolid at 2 and 5 h, and the other group was given PBS. At 0, 8, 12, and 20 h after infection, three mice from each group were imaged and sacrificed, and the bacteria recovered from the catheters were quantified. Both treated and control mice had comparable luminescence (Fig. 6A) and bacterial counts (Fig. 6B), immediately after infection (0 h) and at 8 and 12 h after infection. A decrease in both luminescence (Fig. 6A) and bacterial counts (Fig. 6B) was observed in treated mice at 20 h. Although diminished, the luminescence in treated mice 20 h after infection was still detected (above the background level of uninfected mice) (Fig. 6A).
FIG. 6.
Effects of linezolid on bioluminescence and bacterial counts in the foreign-body model during the first 24 h of infection. (A) Luminescence (in relative light units [RLU]) of linezolid-treated (black circles) or control (white circles) mice. Mice with implanted catheters were infected with S. aureus Xen8.1, given linezolid or PBS p.o., and imaged at 0, 8, 12, and 20 h. Uninf., uninfected. (B) Bacteria recovered from the catheters of linezolid-treated (black circles) and control (white circles) mice. Mice with implanted catheters were infected with S. aureus Xen8.1, given linezolid or PBS p.o., and sacrificed at 0, 8, 12, and 20 h.
Effect of the antibacterial agent linezolid on S. aureus in the deep-wound model.
Treatment with linezolid as a model compound was also used to evaluate the deep-wound infection model. To determine the minimal number of treatments required to clear the infection, in vivo bacterial growth was also monitored over time after treatment with different dosing regimens.
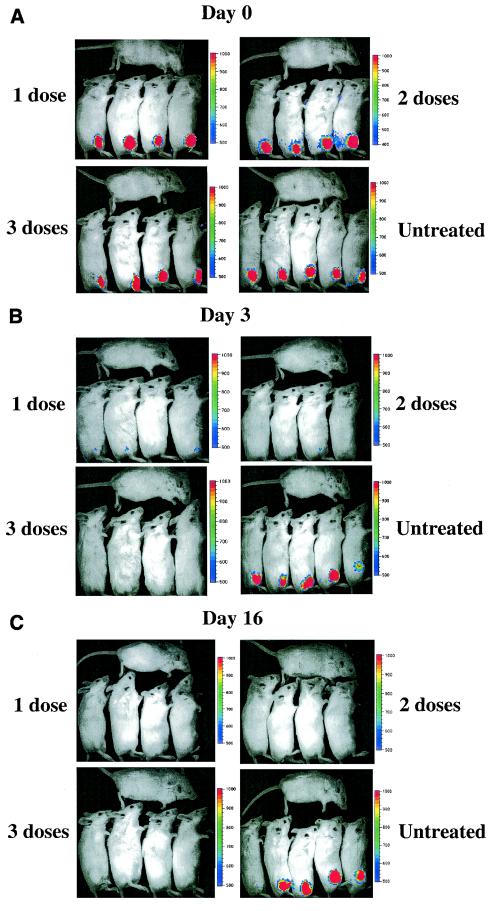
Mice were infected with S. aureus Xen8.1 (108 CFU), randomly assigned into four groups, and imaged. Immediately after infection, all groups had comparable levels of luminescence (Fig. 7A), demonstrating that all mice were infected. Subsequently, the mice were treated either once, twice, or three times with linezolid p.o. As a negative control, a group of infected mice were given PBS once, twice, or three times. No luminescence was detected at 24 h following infection in any of the treated groups (data not shown). However, a low luminescence signal was detected at day 3 for three mice in the single-dose treatment group and for one mouse in the group treated twice (Fig. 7B). The signal was no longer detectable at day 6 (data not shown) or 16 (Fig. 7C). These results suggest that the treatment reduced the bacterial load in these animals to a level below the limit of detection but did not completely clear the infection. Mice treated with three doses of the antibiotic cleared the luminescence at 24 h and showed no subsequent reappearance of luminescence signal through the end of the experiment (day 16 when the experiment was terminated), suggesting complete clearance of the infection in these mice. When the experiment was terminated, all mice were sacrificed, the thighs were collected, and the number of bacteria per thigh were determined. The thighs from mice treated with three doses contained no bacteria, whereas the thighs from untreated mice contained the highest number of bacteria (GMT CFU/thigh, 1.5 × 107 [range, 5.5 × 106 to 8.2 × 107]) (Fig. 8). Mice that received one dose (GMT CFU/thigh, 3.3 × 102 [range, 5 × 101 to 6 × 103]) or two doses (GMT CFU/thigh = 7.7 × 101 [range, 5 × 101 to 2 × 102]) had decreased levels of bacteria recovered from their thighs than the mice in the control untreated group (Fig. 8). The differences between the treated and untreated groups of mice were statistically significant (P < 0.05).
FIG.7.
Complete resolution of luminescence in the deep-thigh-wound model following linezolid treatment of mice infected with S. aureus Xen8.1. BALB/c mice were infected in the thigh with 108 CFU of S. aureus Xen8.1 and subsequently were randomly assigned into five groups. The groups were treated with antibiotic or were given PBS and used as controls as described in Materials and Methods. All mice were imaged at day 0 (after the infection before treatment) and at days 1, 3, 6, and 16. The figure shows mice at days 0 (A), 3 (B), and 16 (C).
FIG. 8.
Resolution of bacterial infection from the thighs of mice infected with S. aureus Xen8.1 and treated with linezolid. Four groups of BALB/c mice were infected in the thigh with 108 CFU of S. aureus Xen8.1. Groups were treated once (at 2 h), twice (at 2 and 5 h), or three times (at 2, 5, and 24 h) with linezolid as described in Materials and Methods. At day 16, all mice were sacrificed, the thighs were removed and ground, and the bacterial titers from the suspensions of cells from individual thighs were determined. The bacterial counts (CFU/thigh) (black circles) and the geometric mean values (black bars) are shown. The P values for the untreated and treated mice were as follows: P = 0.04 for mice treated once, P = 0.03 for mice treated twice, and P < 0.00001 for mice treated three times.
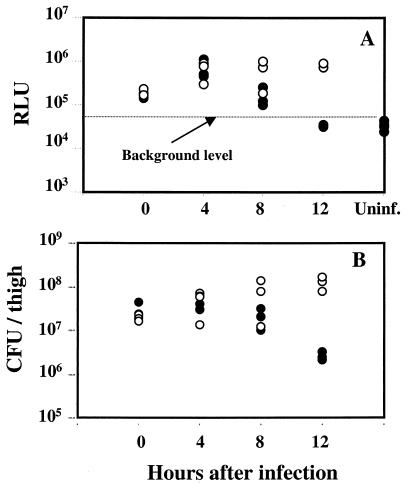
To investigate the effect of linezolid in the deep-wound model during the first 24 h, mice were infected with S. aureus Xen8.1, and half of the mice were treated at 2 and 5 h with linezolid. At 0, 4, 8, and 12 h after infection, all mice were imaged and sacrificed, and the bacteria recovered from infected thighs were quantified. Both treated and untreated groups had comparable luminescence (Fig. 9A) and bacterial counts (Fig. 9B), immediately after infection (0 h) and at 4 and 8 h. Disappearance of luminescence (Fig. 6A) and decrease in bacterial counts (Fig. 6B) were observed in treated mice at 12 h.
FIG. 9.
Effects of linezolid on bioluminescence and bacterial counts in the deep-wound model during the first 24 h of infection. (A) Luminescence (in relative light units [RLU]) of linezolid-treated (black circles) or control (white circles) mice. Mice were infected with S. aureus Xen8.1 in the thighs, given linezolid or PBS p.o., and imaged at 0, 4, 8 and 12 h. Uninf., uninfected. (B) Bacteria recovered from the thighs of linezolid-treated (black circles) and control (white circles) mice. Mice were infected with S. aureus Xen8.1, given linezolid or PBS p.o., and sacrificed at 0, 4, 8, and 12 h.
In addition, we quantified the amount of bacteria recovered from the thighs of treated and control mice at 24 h and 72 h after infection. Two- and three-log-unit reductions of CFU were detected in treated mice compared to control mice at day 1 and day 3, respectively. At day 1 after infection, the GMT CFU/thigh of the control untreated mice was 1.8 × 108 (range, 1 × 108 to 9.9 × 108) and the GMT CFU/thigh of the treated mice was 2.2 × 106 (range, 6 × 105 to 3 × 106). At day 3, the GMT CFU/thigh of the control untreated mice was 1.6 × 108 (range, 1 × 108 to 2.4 × 108) and the GMT CFU/thigh of the treated mice was 6.6 × 104 (range, 3 × 104 to 1.6 × 105).
DISCUSSION
In this study, we describe the development of two murine models, a foreign-body model and a deep-wound model, using bioluminescent S. aureus Xen8.1 and a specialized low-light imaging system. Contag et al. (8) demonstrated for the first time the possibility of monitoring a disease process in a living animal by using bioluminescent pathogens. These method has been proven to have several advantages over the conventional models (8, 14, 15).
The foreign-body model had previously been developed for use with S. epidermidis and was evaluated using the observation of lesions and the number of CFU of bacteria cultivated from a recovered catheter (22, 23, 28). We demonstrate here that this model can be adapted for use with a bioluminescent S. aureus strain and that the pathology of the infection with S. aureus is similar to that reported for S. epidermidis. Our model was evaluated by using luminescence readouts, quantifying CFU from recovered catheters, and monitoring abscess formation. While abscess formation was detected for only a limited period of time, the luminescence signals persisted throughout the length of the experiment (3 and 21 days, respectively).
The second model that we established for use with the bioluminescent S. aureus strain was the deep-wound model of infection. Deep-wound infections in humans caused by S. aureus are frequently seen in hospitals, and several animal models mimicking these clinical manifestations have been developed (5, 11). Deep-wound infection differs from catheter colonization in that the area is more vascularized in the former model, providing the bacteria with greater access to nutrients, therapeutic agents, and antibodies. Bacteria that adhere to catheters likely have reduced access to vascular material, are often coated in a layer of polysaccharide slime, and can have an altered metabolic rate that makes them less susceptible to antibiotic therapy (9, 10). Here we attempted to improve the existing mouse deep-wound model using bioluminescent bacteria. As with the foreign-body model, we demonstrate that a persistent infection that extended throughout the length of the experiment (16 days) can be achieved and can be noninvasively monitored. In both models, the use of bioluminescent bacteria allows real-time and repeated monitoring of the bacterial infection in vivo, enhancing their potential for testing large numbers of vaccine candidates and evaluating therapeutic approaches for the prevention and treatment of S. aureus infections.
To determine whether these models using bioluminescent bacteria can be helpful in evaluating antibacterial strategies, we used linezolid as a model compound. In the foreign-body model, abscess formation was not observed after linezolid treatment, and there was a significant drop of luminescence for 4 days after the infection, followed by an increase that persisted throughout the course of the experiment. Results from the foreign-body model confirm the clinical data that bacterial colonization of surgical implants are difficult to resolve by antibacterial therapy (18) and often require removal of the implant. In the deep-wound model, linezolid treatment resulted in the complete loss of luminescence and complete clearance of bacteria from the infected thigh, as evidenced by the lack of bacterial counts recovered from the ground tissues at day 16 after infection.
Our results demonstrate that models using bioluminescent bacteria facilitated the monitoring of metabolically active S. aureus over time in the same animal, without the need to sacrifice mice at each time point and therefore requiring smaller numbers of animals per experiment. Both models are capable of detecting antibacterial activity and should prove useful in evaluation of antibiotics and prophylactic vaccines. However, one limitation of both models using S. aureus Xen8.1 is their sensitivity. With this bacterial strain, we can monitor antibiotic therapy to a point, namely, the limit of detection, which is ∼105 CFU for the foreign-body model and ∼106 CFU for the deep-wound model. Because of the low sensitivity of the deep-wound Xen8.1 infection model, it might be difficult to differentiate between a marked bactericidal action and a mild drug. In this case, in addition to monitoring the bioluminescence, sacrificing animals and determining the CFU will be necessary. Further refinements of the model including generation of new strains with higher bioluminescence should increase the sensitivity of the models.
In addition to antibiotic screening, these models could be used to test potential S. aureus vaccine candidates. It is conceivable that the luminescent staphylococcal foreign-body model will be useful in testing active immunization strategies where immunity is induced prior to the bacteria producing a biofilm on the implant, enabling the effective eradication of the infection without the need for antibiotic treatment. Alternatively, the luminescent staphylococcal deep-wound infection model might also be used to screen passively transferred monoclonal antibodies for efficacy in treating a staphylococcal infection.
REFERENCES
- 1.Abb, J. 2002. In vitro activity of linezolid, quinupristin-dalfopristin, vancomycin, teicoplanin, moxifloxacin and mupirocin against methicillin-resistant Staphylococcus aureus: comparative evaluation by the E test and a broth microdilution method. Diagn. Microbiol. Infect. Dis. 43:319-321. [DOI] [PubMed] [Google Scholar]
- 2.Allen, G. P., R. Cha, and M. J. Rybak. 2002. In vitro activities of quinupristin-dalfopristin and cefepime, alone and in combination with various antimicrobials, against multidrug-resistant staphylococci and enterococci in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 46:2606-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderegg, T. R., D. J. Biedenbach, and R. N. Jones. 2002. In vitro evaluation of AZD2563, a novel oxazolidinone, against 603 recent staphylococcal isolates. Antimicrob. Agents Chemother. 46:2662-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergamini, T. M., R. A. Corpus, Jr., T. M. McCurry, J. C. Peyton, K. R. Brittian, and W. G. Cheadle. 1995. Immunosuppression augments growth of graft-adherent Staphylococcus epidermidis. Arch. Surg. 130:1345-1350. [DOI] [PubMed] [Google Scholar]
- 5.Bunce, C., L. Wheeler, G. Reed, J. Musser, and N. Barg. 1992. Murine model of cutaneous infection with gram-positive cocci. Infect. Immun. 60:2636-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns, S. M., D. Joh, K. P. Francis, X. Y. Song, C. A. Gruber, P. R. Contag, and C. H. Contag. 2001. Revealing the spatiotemporal patterns of bacterial infectious diseases using bioluminescent pathogens and whole body imaging. Anim. Test. Infect. 9:71-88. [DOI] [PubMed] [Google Scholar]
- 7.Chambers, H. F. 2001. The changing epidemiology of Staphylococcus aureus? Emerg. Infect. Dis. 7:178-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Contag, C. H., P. R. Contag, J. I. Mullins, S. D. Spilman, D. K. Stevenson, and D. A. Benaron. 1995. Photonic detection of bacterial pathogens in living hosts. Mol. Microbiol. 18:593-603. [DOI] [PubMed] [Google Scholar]
- 9.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. [DOI] [PubMed] [Google Scholar]
- 10.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 11.Craig, W. A., J. Redington, and S. C. Ebert. 1991. Pharmacodynamics of amikacin in vitro and in mouse thigh and lung infections. J. Antimicrob. Chemother. 27:29-40. [DOI] [PubMed] [Google Scholar]
- 12.Easmon, C. S., and A. A. Glynn. 1977. Effect of cyclophosphamide on delayed hypersensitivity to Staphylococcus aureus in mice. Immunology 33:767-776. [PMC free article] [PubMed] [Google Scholar]
- 13.Foster, T. J., and M. Hook. 1998. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 6:484-488. [DOI] [PubMed] [Google Scholar]
- 14.Francis, K. P., D. Joh, C. Bellinger-Kawahara, M. J. Hawkinson, T. F. Purchio, and P. R. Contag. 2000. Monitoring bioluminescent Staphylococcus aureus infections in living mice using a novel luxABCDE construct. Infect. Immun. 68:3594-3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francis, K. P., J. Yu, C. Bellinger-Kawahara, D. Joh, M. J. Hawkinson, G. Xiao, T. F. Purchio, M. G. Caparon, M. Lipsitch, and P. R. Contag. 2001. Visualizing pneumococcal infections in the lungs of live mice using bioluminescent Streptococcus pneumoniae transformed with a novel gram-positive lux transposon. Infect. Immun. 69:3350-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haddadin, A. S., S. A. Fappiano, and P. A. Lipsett. 2002. Methicillin resistant Staphylococcus aureus (MRSA) in the intensive care unit. Postgrad. Med. J. 78:385-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 18.Marrie, T. J., J. Nelligan, and J. W. Costerton. 1982. A scanning and transmission electron microscopic study of an infected endocardial pacemaker lead. Circulation 66:1339-1341. [DOI] [PubMed] [Google Scholar]
- 19.McKenney, D., K. Pouliot, Y. Wang, V. Murthy, M. Ulrich, G. Doring, J. C. Lee, D. A. Goldmann, and G. B. Pier. 2000. Vaccine potential of poly-1-6 beta-D-N-succinylglucosamine, an immunoprotective surface polysaccharide of Staphylococcus aureus and Staphylococcus epidermidis. J. Biotechnol. 83:37-44. [DOI] [PubMed] [Google Scholar]
- 20.Munoz Bellido, J. L., M. N. Gutierrez Zufiaurre, F. J. Sanchez Hernandez, G. Yague Guirao, M. Segovia Hernandez, and J. A. Garcia-Rodriguez. 2002. In vitro activity of linezolid, synercid and telithromycin against genetically defined high level fluoroquinolone-resistant methicillin-resistant Staphylococcus aureus. Int. J. Antimicrob. Agents 20:61-64. [DOI] [PubMed] [Google Scholar]
- 21.Rocchetta, H. L., C. J. Boylan, J. W. Foley, P. W. Iversen, D. L. LeTourneau, C. L. McMillian, P. R. Contag, D. E. Jenkins, and T. R. Parr, Jr. 2001. Validation of a noninvasive, real-time imaging technology using bioluminescent Escherichia coli in the neutropenic mouse thigh model of infection. Antimicrob. Agents Chemother. 45:129-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rupp, M. E., J. S. Ulphani, P. D. Fey, K. Bartscht, and D. Mack. 1999. Characterization of the importance of polysaccharide intercellular adhesin/hemagglutinin of Staphylococcus epidermidis in the pathogenesis of biomaterial-based infection in a mouse foreign body infection model. Infect. Immun. 67:2627-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rupp, M. E., J. S. Ulphani, P. D. Fey, and D. Mack. 1999. Characterization of Staphylococcus epidermidis polysaccharide intercellular adhesin/hemagglutinin in the pathogenesis of intravascular catheter-associated infection in a rat model. Infect. Immun. 67:2656-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sievert, D. M., G. Boulton, D. Stoltman, M. G. Johnson, F. P. Stobierski, P. A. Downes, J. T. Somsel, E. Rudrik, W. Brown, T. Hafeez, E. Lundstrom, R. Flansgan, J. Jonson, J. Mitchell, and S. Chang. 2002. Staphylococcus aureus resistant to vancomycin—United States. Morb. Mortal. Wkly. Rep. 51:565-567. [Google Scholar]
- 25.Snedecor, G. W., and W. G. Cochran. 1974. Statistical methods, 6th ed. Iowa State University Press, Ames.
- 26.Song, X. Y., F. Fox, M. A. Gallo, A. Rosenberg, R. Jordan, D. Shealy, and C. Wagner. 2002. Effects of 2 different anti-tumor necrosis factor-alpha agents in a primate model of subcutaneous abscess formation. J. Infect. Dis. 185:204-213. [DOI] [PubMed] [Google Scholar]
- 27.Wenzel, R. P., and M. B. Edmond. 2001. The impact of hospital-acquired bloodstream infections. Emerg. Infect. Dis. 7:174-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Widmer, A. F., R. Frei, Z. Rajacic, and W. Zimmerli. 1990. Correlation between in vivo and in vitro efficacy of antimicrobial agents against foreign body infections. J. Infect. Dis. 162:96-102. [DOI] [PubMed] [Google Scholar]