Abstract
Insects are easily distinguishable by the absence of legs on the adult abdomen. Studies performed on the Dipteran, Drosophila melanogaster, indicate that this is because of the repressive effects of the homeotic genes Ultrabithorax (Ubx) and abdominal-A (abd-A) on the limb promoting gene Distal-less (Dll) during embryonic development. However, in many species appendage-like structures are present on abdominal segments in embryonic and juvenile stages. Here, by using classical genetics and double-stranded RNA-mediated gene silencing in the red flour beetle, Tribolium castaneum, a species that develops an appendage on the first abdominal segment, we investigate the roles of Ubx and Abd-A in abdominal limb development. We find that in Tribolium, Abd-A, but not Ubx, represses early expression of Dll in the embryonic abdomen. Ubx appears to modify the A1 appendage. This difference in the activities of Abd-A and Ubx is critical for proper development of this appendage. We suggest that an ancestral role of Abd-A in insect abdominal appendage development was in the repression of Dll initiation and that of Ubx was in modulation of abdominal appendage morphology.
An incredible array of morphological variation has arisen during the course of insect evolution. This variation has occurred on a conserved body plan of 6 head segments, 3 thoracic segments, and between 8 and 11 abdominal segments (1). Molecular and genetic analyses of Drosophila development have shown that segmental character is largely under the regulation of the homeotic selector genes (Hox) within the bithorax and Antennapedia gene complexes (2). Comparative analyses suggest that Hox gene expression patterns are largely conserved among all insects (3–6). A central question concerning insect evolution is how morphological variation arose within the conserved environment of Hox gene expression.
A defining character of the insect body plan is the lack of appendages on the adult abdomen. In the Dipteran Drosophila melanogaster, and likely in the Lepidopteran Precis coenia, suppression of abdominal legs occurs through the repression of Distal-less (Dll) expression by members of the bithorax complex (7–9). Detailed studies performed on Drosophila have revealed that the regulation of Dll expression occurs in two stages. First, the early Dll enhancer is activated by the intersection of dorsal/ventral and anterior/posterior signaling molecules (10). This activating signal is present in all segments. However, in the abdomen, the early promoter is silenced by the presence of Ultrabithorax (Ubx) and abdominal-A (Abd-A) proteins (7, 8). Later, Dll expression is driven by a late enhancer. This enhancer is Dll-dependent and Ubx/Abd-A-independent. In the third thoracic leg, where Ubx and Dll are coexpressed, Dll must be expressed before Ubx to activate the late promoter. Premature expression of Ubx in this segment represses Dll expression (8).
Previous studies on the roles of Hox control of abdominal limb repression in other insect species have focused on comparative expression analysis of Ubx, Abd-A, and Dll during embryonic development (3–6). These studies have relied on a polyclonal antibody that recognizes Dll proteins in a wide range of metazoans, and a monoclonal antibody that recognizes both Ubx and Abd-A proteins in all arthropods examined (11, 12). In contrast to the Dipteran D. melanogaster and the Lepidopteran P. coenia, Ubx/Abd-A proteins are coexpressed with Dll early in the development of embryonic abdominal appendages in species within the lower insect orders Collembola, Orthoptera, and Coleoptera (4). Therefore, the roles of Ubx/Abd-A in regulating abdominal limb repression or development in lower insect orders remains unclear. Previous authors have suggested that Ubx/Abd-A gained their roles as Dll repressors late in insect evolution, with either Abd-A (4) or Ubx (13) evolving limb repressive functions first. Alternatively, it was proposed that segmental differences in Hox gene function, either through the variation of levels of Hox gene expression themselves or in the distribution of Hox cofactors, allows abdominal appendage formation (4).
Here, we examine the roles of the Ubx and abd-A orthologs in the repression and regulation of abdominal appendages in the Coleopteran, Tribolium castaneum (Tc). The Coleoptera comprise a basal lineage within the holometabola. This allows the possibility of establishing polarity of Hox gene evolution in limb repression. In addition, beetles develop pleuropodia, A1 appendages that have been conserved among most insect orders, but lack larval appendages on the more posterior abdominal segments A2–A8. Tribolium offers the ability to perform genetic and reverse genetic experiments not easily performed in other insects outside of Drosophila (14–24). We can therefore investigate the role of the Ubx and abd-A orthologs, independently and together, in regulating abdominal limb development in a basal holometabolous insect.
Analyses of TcUbx and Tcabd-A transcripts using in situ mRNA hybridization have shown that each is expressed in patterns similar to their Drosophila counterparts (18–20). One exception is the detection of TcUbx transcripts earlier and more anterior in the thorax of the beetle relative to Ubx in Drosophila (20, 25, 26). The Ubx and abd-A orthologs have been genetically identified in Tribolium through mutant alleles at the Ultrathorax (Utx) locus and the Abdominal (A) locus, respectively (15, 18–20). In Utx mutant larvae, the pleuropodia develop abnormally and remain visible on the larva. These appendages are smaller than thoracic legs and bear a subterminal tarsal claw (20). Putative null A alleles produce embryos that bear pleuropodial-like appendages on A1–A8. In addition, A mutant larvae bear a protrusion in the posterior third of each abdominal segment (19).
In this report, we analyze the expression of TcUbx, TcAbd-A, TcDll, and TcEn (engrailed) in wild-type and in TcUbx and Tcabd-A mutant embryos to determine what role Ubx and Abd-A play in abdominal appendage development. Using double-stranded RNA interference (RNAi), we also analyze the phenotype of Ubx and abd-A double mutant larvae. These data indicate that in beetle embryos, TcUbx and Tcabd-A have distinct roles in embryonic abdominal limb development. We find that, in the developing pleuropod of the first abdominal segment, TcUbx alone acts to modify appendage morphology in the anterior compartment, whereas TcAbd-A represses appendage outgrowth in the posterior compartment. The presence of TcAbd-A in both the anterior and posterior compartments in more posterior abdominal segments represses all abdominal appendage outgrowth. This is accomplished at least in part through the repression of TcDll. We suggest that an ancestral role of Abd-A in insect abdominal appendage development was in the repression of Dll initiation and that of Ubx was in modulation of abdominal appendage morphology.
Materials and Methods
Beetle Rearing.
Wild-type and mutant strains of T. castaneum were maintained in whole-wheat flour supplemented with 5% brewer's yeast at 26°C. UtxM115 and A10 chromosomes are kept as balanced stocks in trans with Ey, a balancer chromosome carrying a dominant cuticle marker. For embryo collections, adult beetles were transferred to Gold Medal flour supplemented with 5% brewers yeast and mated en masse at 32°C. Eggs were collected from the flour with a fine sieve after 3 days for immunohistochemistry or after 1 h for RNAi experiments.
Immunohistochemistry.
Tribolium eggs were collected, dechorionated, and fixed according to established protocols (27, 28). The eggs were rehydrated stepwise into PBS/0.1% Tween-20 (PBSTw), and the embryonic membranes were dissected away. The embryos were washed and blocked in PBSTw/1% BSA for 30 min at room temperature. For single antibody staining experiments, the cross-reactive rabbit polyclonal antisera raised against Dll was used at a dilution of 1/50 (29). For the double antibody staining experiments, α-Dll antibody was mixed to a final dilution of 1/50 with either a 1/5 dilution of FP6.87, a Ubx/Abd-A specific cross-reactive mouse monoclonal antibody (11), or a 1/5 dilution of Mab4D9, an En-specific cross-reactive mouse monoclonal antibody (27). All 1° antibody incubations were carried out at 4°C overnight. After incubation, the samples were washed at room temperature three times for 5 min each then three times for 15 min each in PBSTw/1% BSA. Then, 2° antibodies were added and incubated overnight at 4°C. In the single-labeling experiments, the horseradish peroxidase (HRP)-conjugated goat α-rabbit 2° antisera (The Jackson Laboratory) was used at a final dilution of 1/500. The HRP color reaction was developed with nickel-enhanced diaminobenzidine substrate. In the double-labeling experiments, fluorescent detection was used. Cy3-conjugated goat α-mouse (The Jackson Laboratory) and fluorescein-conjugated goat α-rabbit antisera (The Jackson Laboratory) were mixed and used at a final dilution of 1/200.
Double-Stranded RNA Interference.
Plasmids containing either a TcUbx or Tcabd-A cDNA were treated with proteinase K and extracted with phenol. These plasmids have been previously described (18, 20). Plasmids were linearized with XhoI or NotI (TcUbx) and XhoI or BamHI (Tcabd-A). Sense and anti-sense transcripts were generated by using 1 μg linearized template and either T3 or T7 RNA polymerases. Transcription reactions were carried out for 2 h in the presence of 40 units RNasin, then treated with DNase1 for 20 min. Reaction products were extracted with phenol and precipitated with ethanol. RNA was resuspended in 20 μl 0.1× PBS, and duplexes were made by mixing equal volumes of complementary RNA, heating to 80°C for 5 min, then allowing the mixture to cool slowly to room temperature. This double-stranded RNA was injected directly into 1- to 2-h-old Tribolium embryos.
Embryo Injections.
Tribolium eggs were collected from a 1-h oviposition of a large-scale wild-type (Ga-1) population. Eggs were treated in 2% bleach for 2 min, washed extensively in dH2O, and mounted on the edge of a glue-treated glass coverslip. Submerged eggs were injected at a setting of 25 psi for 40–60 ms with a Narishige microinjector. After injections, the dH2O was immediately removed from the embryos and the coverslips were placed on apple-juice agar plates (30); these were placed in humidified Petri dishes and incubated at 26°C for 5–6 days. Embryo survival rate averages were approximately 20%, with 80–90% of developing embryos showing discernable homeotic phenotypes.
Preparation of Larvae for Scanning Electron Microscopy (SEM).
Five- to six-day-old hatched and unhatched larvae were collected from coverslips and fixed as follows: hatched larvae were placed directly into 33% dimethylpropane in ethanol for 24+ h at 4°C, washed three times in ethanol, and critical point dried for SEM. Unhatched larvae were dissected off the coverslips, placed into Superskipper solution (30) for 30–90 s, then transferred to Carl's Fixative for 24–48 h at 4°C (30). The animals were washed five times in ethanol and critical point dried for SEM.
Cuticle Preparation.
Larval cuticles were prepared for fluorescence microscopy by using the method of van der Meer (31).
Microscopy.
For light microscopy, embryos were mounted in 80% glycerol and imaged using Namarski optics on a Zeiss Axiophot. For confocal microscopy, samples were mounted in Vectashield (Vector Laboratories) and imaged with a Nikon Optiphot and Bio-Rad MRC 1024 laser. SEM was carried out on a Hitachi S-570 scanning electron microscope.
Results
The Pleuropod Is an Anterior Compartment-Specific Appendage.
To gain a better understanding of pleuropod development and the potential interactions among TcDll, TcUbx, and TcAbd-A, we followed the expression of these proteins during embryogenesis in wild-type animals (Fig. 1). The interspecific cross-reactive antibody Mab4D9 that detects the Tribolium En protein, was used as a marker for the posterior compartment of each segment (28). In animals stained for TcDll and TcEn expression, two observations are relevant. First, TcDll- and TcEn-expressing cells within the A1 segment are completely exclusive of one another. Thus, the distal outgrowth of the pleuropodia is derived from non-En-expressing cells. This differs from the thoracic appendages (legs), where cells in the posterior coexpress TcDll and TcEn (Fig. 1 A–D). Second, TcDll-expressing cells initially have the appearance of the normal epithelial cells of the thoracic legs (Fig. 1 A and B); however, later in development, the nuclei of TcDll-expressing cells become distinctly larger and the cells less packed (Fig. 1 C and D).
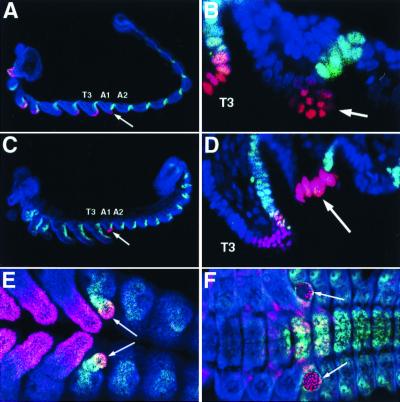
Figure 1.
Embryonic expression patterns of TcDll, TcEn, and TcUbx/Abd-A during pleuropod development. (A–D) Histochemical labeling to visualize TcDll (red) and TcEn (green) in developing beetle embryos. An arrow indicates the pleuropod. Anterior is toward the left and ventral is toward the bottom. (A and B) Confocal micrographs of an early germ-band-extended embryo at low magnification (A) and at high magnification, showing the pleuropod and T3 leg (B). At this stage of development, the pleuropod contains TcDll-expressing cells, which are similar in size and shape to those of the embryonic legs. However, unlike in the leg, none of these cells coexpress the TcEn protein, indicating that at least the distal part of the pleuropod is entirely within the anterior compartment of the A1 segment. (C and D) Later in development, the pleuropod begins to invaginate, and the distal-most cells become enlarged and express high levels of TcDll protein. (E and F) Histochemical labeling to visualize TcDll (red) and TcUbx/Abd-A (green) protein. Views are ventral, and anterior is toward the left. (E) TcUbx/Abd-A protein is coexpressed with TcDll protein in the pleuropod during germ-band extension (arrows). The staining here is most likely because of TcUbx alone, as TcAbd-A is not expressed anterior to A1p. (F) During germ band retraction, TcUbx is no longer expressed in the enlarged TcDll-expressing cells in the pleuropod. All embryos were counterstained with the nuclear dye ToPro-3 (blue). T3, third thoracic segment; A1, first abdominal segment; A2 second abdominal segment.
Using an antibody that crossreacts with Ubx/Abd-A proteins, Palopoli and Patel (4) showed that the embryonic expression for TcUbx/Abd-A occurs concomitant with TcDll expression during early pleuropod development. During early pleuropod development. our results confirmed that of Palopoli and Patel (Fig. 1E). However, later in development, the TcDll-expressing cells no longer express detectable levels of TcUbx/Abd-A (Fig. 1F). At this point, TcUbx/Abd-A expression is limited to the more proximal regions of the pleuropod.
Regulation of Pleuropod Development by TcUbx.
The data presented above suggests that the distal outgrowth of the pleuropod develops entirely within the anterior compartment of A1. Wild-type expression patterns of TcUbx and TcAbd-A indicate that only TcUbx is expressed in this compartment (18–20). Previously reported alleles of Utx cause transformation of the A1 pleuropodia toward thoracic appendage. In addition, larvae that are homozygous or hemizygous for these alleles lack the A1 spiracle. As wild-type Tribolium larvae have spiracles in T2 and all abdominal segments, but lack spiracles in the T1 and T3 segments, it was concluded that these larvae had transformations of the A1 segment toward the T3 segment (or more precisely, ps6 to ps5) (20). A recently isolated Utx allele, Utxm115 shows a more complete homeotic transformation. Homozygous Utxm115 larvae show the presence of an A1 appendage, indicative of Utx mutations, but, in addition, spiracles are now present in the anterior third of both T3 and A1 (data not shown). We interpret this as transformation of the T3/A1 segments toward the T2 segment (or again, more precisely, ps5 and 6 to ps4), the more expected phenotype of a Utx null. To determine whether Utxm115 is a protein null, we stained Utxm115 embryos with anti-Ubx/Abd-A antibody. In these embryos, the Ubx/Abd-A crossreactive antibody fails to detect protein anterior to A1p (ps7, Fig. 2F), suggesting that Utxm115 is a protein null or nearly so.
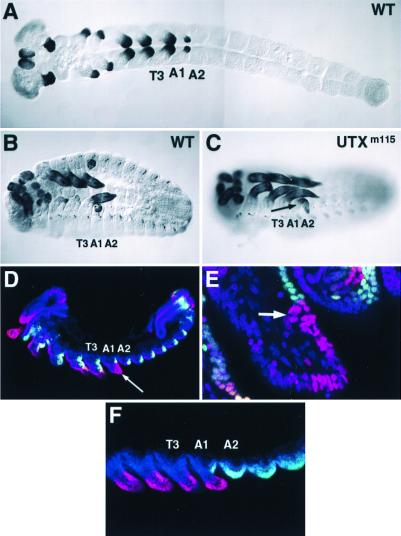
Figure 2.
Pleuropod development is altered in Utxm115 mutant embryos. (A–C) Histochemical labeling showing TcDll expression in an early germ-band-extended stage wild-type embryo (A), a germ-band-retracted stage wild-type embryo (B), and a germ-band-retracted stage Utxm115 mutant embryo (C). The views are ventral, and anterior is toward the left. In the Utxm115 embryo, the pleuropod (arrow) never invaginates and instead is transformed to an appendage on the A1 segment that remains external throughout embryonic development. (D and E) Histochemical labeling of Utxm115 embryos for Tc Dll (red) and TcEn (green). Embryos were counterstained with the nuclear dye ToPro-3 (blue). Anterior is toward the left, and ventral is toward the bottom. (D) Low magnification confocal micrograph of the transformed A1 appendage. The appendage (arrow) is increased in size and contains a greater number of TcDll-expressing cells when compared with the pleuropod at a similar stage of development in wild-type embryos (compare with Fig. 1D). (E) High magnification confocal micrograph of the transformed A1 appendage. The TcDll-expressing ectodermal cells in the transformed A1 appendage are similar in size and shape to the ectodermal cells of thoracic legs, and do not become enlarged as in the pleuropod of the wild type. TcEn expression is detected in ectodermal cells in the posterior of the appendage extending from the base of the appendage to a point near the middle of the appendage and abutting TcDll-expressing cells. As in the wild-type pleuropod, no cells are seen to coexpress TcDll and TcEn. At the boundary of TcEn-expressing cells, Dll is expressed in a group of cells (arrow). This domain correlates with the position of the subterminal claw in a Utxm115 larva or in animals injected with TcUbx-RNAi (see Fig. 4C). (F) Histochemical labeling of Utxm115 mutant embryos for TcDll (red) and TcUbx/Abd-A (green). The absence of labeling anterior of PS6 (A1a) suggests that Utxm115 is a TcUbx protein null, therefore the labeling observed is likely because of TcAbd-A alone. As for TcEn, no cells are seen to coexpress TcDll and TcAbd-A in the A1 appendage.
We followed TcDll expression in Utxm115 embryos to determine what role TcUbx may be playing in regulating TcDll expression and pleuropod development in the abdomen (Fig. 2). In Utxm115 embryos, TcDll expression in the head and thoracic appendages appears wild type (Fig. 2 C and D). Within the transformed A1 segment, no detectable phenotype could be discerned early. However, as the A1 appendage developed, the TcDll-expressing domain expanded (Fig. 2 D–F). In addition, the nuclei of the TcDll-expressing cells never developed the characteristic morphology of the pleuropodia, and instead remained small in size, similar to those in the leg (Fig. 2E). As in the wild type, TcDll expression is restricted to the anterior compartment, as evidenced by the lack or TcEn-staining in these cells. Interestingly, the junction between the TcEn expression and TcDll expression occurs near the midpoint of the appendage and appears to be at or near the location of the subterminal tarsal claw in the first instar larva of Utx mutants (compare with Fig. 4C). This position may correspond to the true distal tip of the appendage. Ectopic TcDll expression was not observed in A1p and more posterior abdominal segments, suggesting that TcAbd-A alone is sufficient to repress TcDll in these segments. In contrast, TcUbx appears to affect the final morphology of the pleuropod and does not appear to have a role in repressing initial TcDll expression in the abdomen.
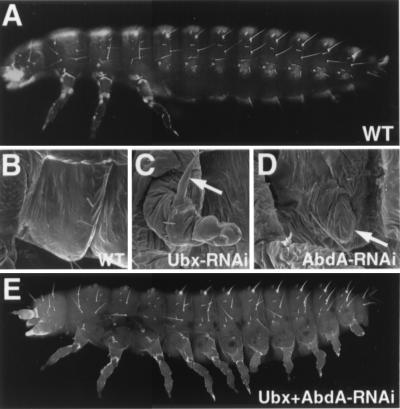
Figure 4.
dsRNA-mediated interference of TcUbx and Tcabd-A expression results in the production of legs on abdominal segments. (A) Fluorescent micrograph of the cuticle of a wild-type first instar larva. Note the absence of any appendages on the abdomen. (B–D) SEM showing the ventro-lateral aspect of the A1 segment in a larval-stage wild-type animal (B), a larval-stage animal that had been injected during embryogenesis with TcUbx-RNAi (C), and a larval-stage animal that had been injected during embryogenesis with Tcabd-A-RNAi. In the wild type, the cuticle of the A1 segment is smooth and no outgrowth is visible. In the TcUbx-RNAi injected animal, an appendage is present on the transformed A1 segment that is similar in appearance to that observed in a Utxm115 mutant. Note the subterminal claw located roughly at the midpoint of the proximal-distal axis (arrow). In the Tcabd-A-RNAi animal, a small outgrowth is observed on the transformed A1 segment (arrow) as well as on the more posterior abdominal segments (not shown). (E) Fluorescent micrograph of the cuticle of a first instar larva injected with TcUbx-RNAi and Tcabd-A-RNAi. In this animal, thoracic-like limbs are present on the transformed abdominal segments A1–A8.
TcAbd-A Represses TcDll Expression.
The lack of overlap between TcAbd-A and TcDll protein expression suggests that TcAbd-A may repress TcDll in the beetle abdomen as it does in Drosophila. Indeed, embryos homozygous for mutant alleles of Tcabd-A contain ectopic pleuropodia throughout the abdomen, and, in the larvae, there is a protrusion in the posterior third of each abdominal segment (Fig. 3C, and ref. 19). We therefore examined TcDll expression in Tcabd-A mutant embryos. In homozygotes of a putative null allele of Tcabd-A, A10 (19), TcDll was expressed in patches of cells in the transformed abdominal segments A1–A8 (Fig. 3B). In later embryos, two distinct domains of TcDll expression within each segment were apparent (Fig. 3 C–E). In the anterior two-thirds, the nuclei had the characteristic pleuropodial morphology (large dispersed nuclei). In the posterior third of each segment, the nuclei remain small and more densely packed and are similar in appearance to nuclei of TcDll-expressing cells in the leg. To determine whether these two distinct cell types were contained within separate compartmental boundaries, we stained A10 mutant embryos for TcDll and TcEn proteins. We found no coexpression of TcDll and TcEn in the large nuclei characteristic of distal pleuropod nuclei in the anterior two-thirds of the appendage, however TcDll and TcEn were coexpressed in the smaller nuclei in the posterior one-third of the appendage (Fig. 3E). These results indicate that, in wild-type embryos, TcAbd-A represses TcDll expression in the abdomen.
Figure 3.
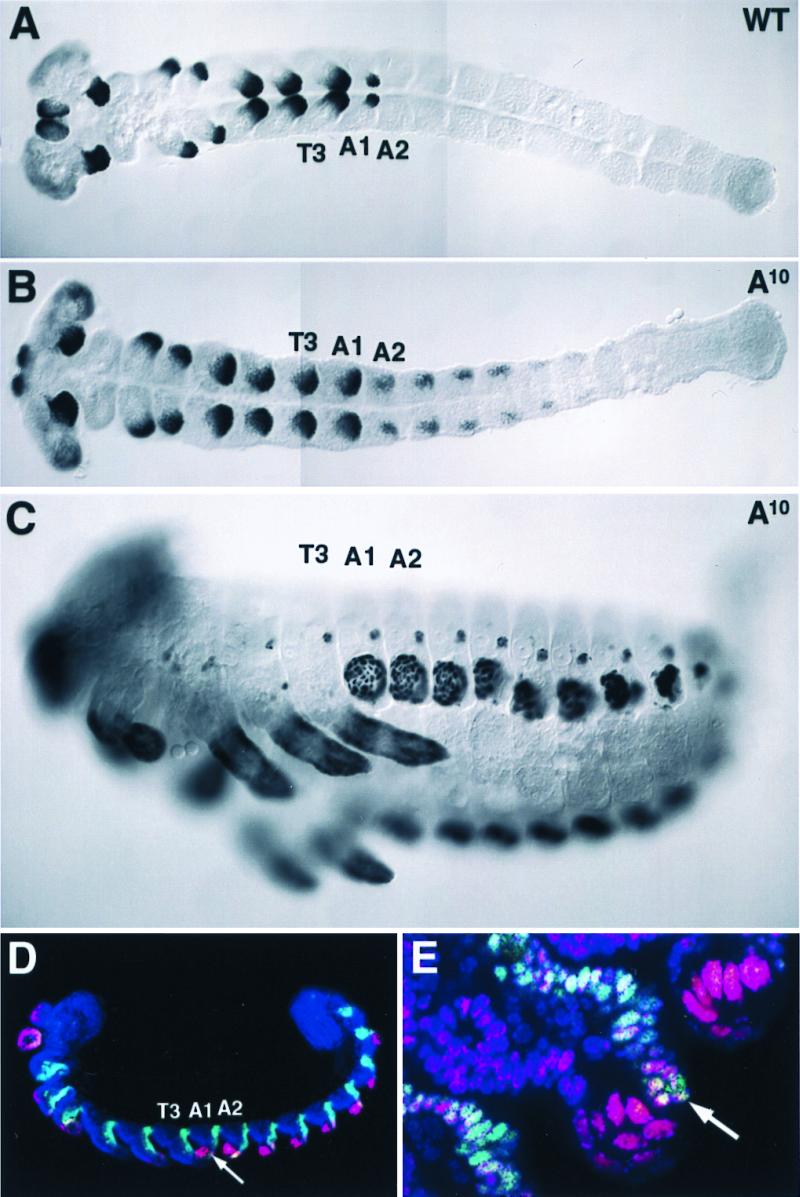
TcDll and TcEn expression overlap in abdominal appendages of A10 mutant embryos. (A–C) Histochemical labeling of TcDll in an early germ-band-extended stage wild-type embryo (A), an early germ-band-extended stage A10 mutant embryo (B), and a germ-band-retracted A10 mutant embryo (C). The views are ventral, and anterior is toward the left. In wild-type embryos, abdominal expression of TcDll is only observed in the pleuropod, whereas TcDll-expressing cells are observed in patches of cells in the transformed A1–A8 segments in the A10 mutant. During germ-band retraction, the large TcDll-expressing cells in the abdominal segments of the A10 mutant are similar in appearance to the Dll-expressing cells of the pleuropod in the wild type (compare with Fig. 2B). (D and E) Histochemical labeling of TcDll (red) and TcEn (green). Embryos were counterstained with the nuclear dye ToPro-3 (blue). Anterior is toward the left, and ventral is toward the bottom. (D) Low magnification confocal micrograph showing TcDll-expressing cells in the appendages of A1–A8 segments. TcEn overlaps with the posterior-most TcDll-expressing cells (arrow). (E) High magnification confocal micrograph of the A1 appendage. Just posterior to the large, pleuropod-like TcDll-expressing cells, smaller TcDll-expressing cells (arrow) of similar size and shape to TcDll-expressing cells in thoracic legs are observed. These cells are located in the posterior compartment of the limb, as evidenced by the coexpression of TcEn.
Targeted Disruption of TcUbx and Tcabd-A Expression Allows Leg Development on Abdominal Appendages.
The results above suggest that TcUbx, when expressed in the anterior compartment of an abdominal appendage, imparts pleuropod identity. Expression of TcUbx in the posterior compartment in the absence of TcAbd-A promotes leg development. This implies that, in animals singly mutant for either TcUbx or Tcabd-A, legs cannot form on abdominal segments because posterior compartment cells in TcUbx mutants contain TcAbd-A, which represses TcDll, and anterior compartment cells in Tcabd-A mutants express TcUbx, which promotes pleuropod fate. A lack of coordinated growth and gene expression between anterior and posterior compartments of the appendage would prevent properly patterned legs from developing on the abdomen. A prediction that arises is that animals mutant for both TcUbx and Tcabd-A would develop legs on each abdominal segment. To test this, we removed both gene functions simultaneously using RNAi. RNAi was prepared for each gene and injected singly or together into preblastoderm eggs. Eggs injected singly with either TcUbx-RNAi or Tcabd-A-RNAi produced larvae that phenocopied the presumptive null condition for each gene (Fig. 4 C and D). When a 1:1 mixture of both RNAis was injected, larvae were produced with nearly wild-type thoracic legs on the transformed segments A1–A6. The legs on the more posterior segments (A7 and A8) were not quite as fully developed, but leg-like features such as joints could be recognized. These data indicate that legs result only in the absence of both the limb modifier TcUbx and the limb repressor TcAbd-A.
Discussion
Roles of Ubx and Abd-A in Regulating Pleuropod Development.
In this report, we sought to elucidate the roles of Ubx and abd-A orthologs in regulating abdominal appendage development in a basal holometabolan lineage. By examining TcDll and TcEn expression in TcUbx and Tcabd-A mutant embryos, we were able to gain a better understanding of the role of each in suppressing and modifying limb programs in the beetle abdomen. In TcUbx mutant embryos, TcDll expression in the abdomen remained restricted to anterior A1, whereas, in Tcabd-A, mutant embryo TcDll was ectopically expressed in each abdominal segment, resulting in abdominal appendage development. These results clearly support the role for TcAbd-A as a primary TcDll repressor (and therefore appendage repressor) in the Tribolium abdomen. The role of TcUbx in regulating Dll expression appears to be more complex. Although TcDll and TcUbx are initially coexpressed during early pleuropod development, later TcUbx is absent in the TcDll-expressing cells, leaving open the possibility that TcUbx represses TcDll late in development. Whether or not late expression of TcUbx represses TcDll expression in these cells, it is evident from mutant analysis that TcUbx is required for the proper differentiation of these cells. In TcUbx mutants, the nuclei of TcDll-expressing cells in the pleuropod never become morphologically distinct as they do in the wild type. We therefore believe that TcUbx acts as a modifier rather than a repressor of abdominal appendage development.
The dynamic relationship between TcUbx and TcDll expression in the pleuropod and the effect of TcUbx expression on the differentiation of TcDll-expressing cells suggests that TcUbx acts to modify the way cells in the anterior A1 compartment interpret signaling cues. In the absence of TcUbx, cells respond to signaling cues as if they were no longer pleuropodial. The failure of the appendage to invaginate and the presence of the subterminal tarsal claw in TcUbx mutant larvae support this view. In addition, the position of the subterminal tarsal claw appears to correspond to the boundary of TcEn expression and the cluster of TcDll-expressing cells in the developing appendage of the embryo. We interpret this as evidence that these cells now respond to signaling cues as if they were leg, with the distal-most tip, the tarsal claw in the leg, at the intersection of the anterior–posterior boundary.
Differences in the manner in which TcUbx-expressing cells respond to signaling cues could be because of TcUbx acting directly on signaling pathway components or their targets. Studies performed on Ubx control of wing vs. haltere development in Drosophila have indeed shown that Ubx can act at multiple levels of a genetic hierarchy (32). In the case of pleuropod development, the levels of Ubx and/or the presence of Hox cofactors are likely to be responsible for pleuropod-specific gene expression. We favor the former explanation as very high levels of TcUbx are found in the pleuropod compared with the levels found in other regions of the embryo. The levels of TcUbx expression may be important to outcompete other proteins expressed in these cells, such as Antennapedia, which normally promote leg patterning (33, 34). In addition, it has been shown that TcUbx levels are decreased in TcEn-expressing cells of the thorax and abdomen in wild-type embryos (20). Differences in TcUbx levels in these compartments may also explain why, in Tcabd-A mutants, only the cells in the anterior compartment of the abdominal segments are able to differentiate as pleuropodial cells, whereas the TcEn-expressing cells in the posterior compartment differentiate as leg cells. The possible effect of Ubx levels on pleuropod patterning is consistent with data obtained in Drosophila on the effects of Ubx levels on patterning ps6 in the embryo and bristles on the T2 leg in the adult (35–38).
Evolutionary Considerations.
Comparing the data obtained in this study on beetle abdominal appendage development with that obtained from other holometabolous insects (4, 11, 13, 18, 20), we suggest that abdominal limb repression through direct Abd-A repression of Dll expression evolved at the latest in the last common ancestor of the holometabola. This is the most parsimonious interpretation given that the repressive activity of Abd-A is evident in species from all of the holometabolous orders examined. However, one holometabolous insect species, the Lepidopteran Manduca sexta, appears to be an exception (13). In the developing abdominal prolegs in this species, Dll is expressed despite the coexpression of Ubx/Abd-A. It is interesting to note that the ability to express Dll in developing prolegs has arisen using at least two different mechanisms within the Lepidoptera. In the butterfly Precis coenia, activation of Dll expression in the abdomen is correlated with regional repression of Ubx/Abd-A (6), whereas, in the moth Manduca sexta, Dll expression occurs through a different mechanism, presumably involving the escape of Dll from the repressive effects of Abd-A (13). These data suggest that the release of the repressive effect of Abd-A on abdominal limbs in higher holometabolous insects occurred convergently through changes at different levels of the limb regulatory hierarchy. Alternatively, it is possible, although we consider it less likely, that the regional repression/expression of Ubx/Abd-A has no causative effect on proleg outgrowth, leaving open the possibility that the presence of prolegs in these two Lepidopteran species is not convergent.
In higher holometabolous insect species, such as those found in the orders Diptera and Lepidoptera, Ubx can act as a primary repressor of Dll expression in the abdomen, whereas, in the more basal species such as Tribolium, Ubx acts instead as a modifier of abdominal limb development. Both the modifier role of Ubx in the anterior A1 compartment and the repressive role of Abd-A in the posterior compartment are required for proper pleuropod development in Tribolium. Because pleuropodia develop in the A1 segment of most insect orders (39), we believe limb modification rather than limb repression is a more ancient property of Ubx. Given the conserved expression patterns of Ubx and Abd-A in the insect abdomen, it will be of interest to examine how the functions of these genes in regulating abdominal appendage development have changed during the course of insect evolution.
Acknowledgments
We thank Rob White, Grace Panganiban, and Nipam Patel for providing crossreactive anti-Ubx/Abd-A, -Dll, and -En antibodies, respectively. We thank Sue Brown and Robin Denell for the Abdominal cDNA. We also thank Wendall Burkholder for providing laboratory space for rearing beetles. R.L.B. thanks Sean Carroll for hosting him during much of this work. David L. Lewis is a Research Associate for the Howard Hughes Medical Institute. R.L.B. acknowledges support from the Brigham Young University Professional Development Fund.
Abbreviations
- En
engrailed
- Tc
Tribolium castaneum
- Ubx
Ultrabithorax
- abd-A
abdominal-A
- Dll
Distal-less
- Utx
Ultrathorax
- A
abdominal
- RNAi
interfering RNA
- ps
parasegment
- SEM
scanning electron microscope
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Snodgrass R E. Principles of Insect Morphology. New York: MacGraw–Hill; 1935. [Google Scholar]
- 2.Lawrence P A, Morata G. Cell. 1994;60:181–189. doi: 10.1016/0092-8674(94)90289-5. [DOI] [PubMed] [Google Scholar]
- 3.Averof M, Patel N H. Nature (London) 1997;388:682–686. doi: 10.1038/41786. [DOI] [PubMed] [Google Scholar]
- 4.Palopoli M F, Patel N. Curr Biol. 1997;8:587–590. doi: 10.1016/s0960-9822(98)70228-3. [DOI] [PubMed] [Google Scholar]
- 5.Grenier J K, Garber T L, Warren R, Whitington P M, Carroll S. Curr Biol. 1997;7:547–553. doi: 10.1016/s0960-9822(06)00253-3. [DOI] [PubMed] [Google Scholar]
- 6.Warren R W, Nagy L, Selegue J, Gates J, Carroll S. Nature (London) 1994;372:458–461. doi: 10.1038/372458a0. [DOI] [PubMed] [Google Scholar]
- 7.Vachon G, Cohen B, Pfeifle C, McGuffin M E, Botas J, Cohen S M. Cell. 1992;71:437–450. doi: 10.1016/0092-8674(92)90513-c. [DOI] [PubMed] [Google Scholar]
- 8.Castelli-Gair J, Akam M. Development (Cambridge, UK) 1995;121:2973–2982. doi: 10.1242/dev.121.9.2973. [DOI] [PubMed] [Google Scholar]
- 9.Warren R W, Nagy L, Selegue J, Gates J, Carroll S. Nature (London) 1995;372:458–461. doi: 10.1038/372458a0. [DOI] [PubMed] [Google Scholar]
- 10.Lecuit T, Cohen S M. Nature (London) 1997;388:139–145. doi: 10.1038/40563. [DOI] [PubMed] [Google Scholar]
- 11.Kelsh R, Weinzierl R O, White R A, Akam M. Dev Genet (Amsterdam) 1994;15:19–31. doi: 10.1002/dvg.1020150104. [DOI] [PubMed] [Google Scholar]
- 12.Panganiban G, Irvine S M, Lowe C, Roehl H, Corley L S, Sherbon B, Grenier J K, Fallon J F, Kimble J, Walker M, et al. Proc Natl Acad Sci USA. 1997;94:5162–5166. doi: 10.1073/pnas.94.10.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng Z, Khoo A, Fambrough D, Jr, Garza L, Booker R. Dev Genes Evol. 1999;209:460–472. doi: 10.1007/s004270050279. [DOI] [PubMed] [Google Scholar]
- 14.Stuart J J, Brown S J, Beeman R W, Denell R E. Nature (London) 1991;350:72–74. doi: 10.1038/350072a0. [DOI] [PubMed] [Google Scholar]
- 15.Beeman R W, Stuart J J, Haas M S, Denell R E. Dev Biol. 1989;133:196–209. doi: 10.1016/0012-1606(89)90311-4. [DOI] [PubMed] [Google Scholar]
- 16.Beeman R W, Stuart J J, Brown S J, Denell R E. BioEssays. 1993;15:439–444. doi: 10.1002/bies.950150702. [DOI] [PubMed] [Google Scholar]
- 17.Beeman R W, Stuart J J, Haas M S, Friesen K S. J Hered. 1996;87:224–232. doi: 10.1093/oxfordjournals.jhered.a022989. [DOI] [PubMed] [Google Scholar]
- 18.Shippy T D, Brown S J, Denell R E. Dev Genes Evol. 1998;207:446–452. doi: 10.1007/s004270050135. [DOI] [PubMed] [Google Scholar]
- 19.Stuart J J, Brown S J, Beeman R W, Denell R E. Development (Cambridge, UK) 1993;117:233–243. doi: 10.1242/dev.117.1.233. [DOI] [PubMed] [Google Scholar]
- 20.Bennett R L, Brown S J, Denell R E. Dev Genes Evol. 1999;209:608–619. doi: 10.1007/s004270050295. [DOI] [PubMed] [Google Scholar]
- 21.Brown S, Holtzman S, Kaufman T, Denell R. Dev Genes Evol. 1999;209:389–398. doi: 10.1007/s004270050269. [DOI] [PubMed] [Google Scholar]
- 22.Maderspacher F, Bucher G, Klingler M. Dev Genes Evol. 1998;208:558–568. doi: 10.1007/s004270050215. [DOI] [PubMed] [Google Scholar]
- 23.Sulston I A, Anderson K V. Dev Genet. 1998;23:56–64. doi: 10.1002/(SICI)1520-6408(1998)23:1<56::AID-DVG6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 24.Sulston I A, Anderson K V. Development (Cambridge, UK) 1996;122:805–814. doi: 10.1242/dev.122.3.805. [DOI] [PubMed] [Google Scholar]
- 25.Akam M E. EMBO J. 1983;2:2075–2084. doi: 10.1002/j.1460-2075.1983.tb01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akam M E, Martinez-Arias A, Weinzierl R, Wilde C D. Cold Spring Harbor Symp Quant Biol. 1985;50:195–200. doi: 10.1101/sqb.1985.050.01.025. [DOI] [PubMed] [Google Scholar]
- 27.Patel N H, Martin-Blanco E, Coleman K G, Poole S J, Ellis M C, Kornberg T B, Goodman C S. Cell. 1989;58:955–968. doi: 10.1016/0092-8674(89)90947-1. [DOI] [PubMed] [Google Scholar]
- 28.Brown S J, Patel N H, Denell R E. Dev Genet. 1994;15:7–18. doi: 10.1002/dvg.1020150103. [DOI] [PubMed] [Google Scholar]
- 29.Panganiban G, Sebring A, Nagy L, Carroll S. Science. 1995;270:1363–1366. doi: 10.1126/science.270.5240.1363. [DOI] [PubMed] [Google Scholar]
- 30.Ashburner M. Drosophila: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 31.van der Meer J M. Drosophila Inf Serv. 1977;52:160. [Google Scholar]
- 32.Weatherbee S D, Halder G, Kim J, Hudson A, Carroll S. Genes Dev. 1998;12:1474–1482. doi: 10.1101/gad.12.10.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryoo H D, Mann R S. Genes Dev. 1999;13:1704–1716. doi: 10.1101/gad.13.13.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casares F, Mann R S. Nature (London) 1998;392:723–726. doi: 10.1038/33706. [DOI] [PubMed] [Google Scholar]
- 35.Castelli-Gair J, Greig S, Micklem G, Akam M. Development (Cambridge, UK) 1994;120:1983–1995. doi: 10.1242/dev.120.7.1983. [DOI] [PubMed] [Google Scholar]
- 36.Akam M. Curr Biol. 1998;8:R676–R678. doi: 10.1016/s0960-9822(98)70433-6. [DOI] [PubMed] [Google Scholar]
- 37.Mann R. Development (Cambridge, UK) 1994;120:3205–3212. doi: 10.1242/dev.120.11.3205. [DOI] [PubMed] [Google Scholar]
- 38.Stern D L. Nature (London) 1998;396:463–466. doi: 10.1038/24863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwalm F E. Insect Morphogenesis. New York: Karger; 1988. [PubMed] [Google Scholar]