Abstract
A cyclic polyisoprenoid compound, geranylgeranylacetone (GGA), has been used as antiulcer drug. GGA is also a potent inducer of heat shock proteins (HSPs). HSPs are considered to induce an antiviral effect; however, the detailed mechanism is unknown. To determine whether GGA might show antiviral activity and what the mechanism is, the effect of GGA against influenza virus (strain PR8) infection in vivo and in vitro was investigated. The results demonstrated that GGA treatment strongly suppressed the deleterious consequences of PR8 replication and was accompanied by an increase in HSP70 gene expression in mice. Results from in vitro analyses demonstrated that GGA significantly inhibited the synthesis of PR8-associated proteins and prominently enhanced expression of human myxovirus resistance 1 (MxA) followed by increased HSP70 transcription. Moreover, GGA augmented the expression of an interferon-inducible double-strand RNA-activated protein kinase (PKR) gene and promoted PKR autophosphorylation and concomitantly α subunit of eukaryotic initiation factor 2 phosphorylation during PR8 infection. It is proposed that GGA-induced HSP70 has potent antiviral activity by enhancement of antiviral factors and can clinically achieve protection from influenza virus infection.
Influenza virus causes recurrent epidemics and global pandemics with acute febrile respiratory disease in all age groups. Hospitalization and serious complications are often accompanied by death, especially in children, the elderly, and immune system-compromised hosts (4, 9). Influenza virus, particularly type A, has the potential to evoke a novel mutant virus through genetic reassortment or point mutation. Although inactivated vaccine achieves a certain amount of protection in healthy subjects, it is less effective in elderly patients (26). Amantadine and rimantadine (40) or new neuraminidase inhibitors (10, 12) have been available for therapy or prevention; however, a few adverse effects and the emergence of resistant viral strains have been reported previously (7, 15, 20).
Geranylgeranylacetone (GGA), an acyclic polyisoprenoid compound formulated with a retinoid skeleton, has been developed in Japan to be used orally as an antiulcer drug. It has the ability to protect the gastric mucosa from damage resulting from various stresses and is attracting interest as a heat shock protein (HSP) inducer with its lack of cytotoxicity in possible clinical applications (13, 25, 41). HSPs, most notably HSP70 (with a molecular mass of 70 kDa), are induced intracellularly by a variety of environmental or physiological stresses, such as heat, hypoxia, ischemia, and infection. HSP70 is an integral feature of homeostasis and plays a key role in providing a cytoprotective effect, which suggests that induction of HSP70 can be advantageous to the cell in protection against stressors or diseases. Interestingly, HSP70 induction gives rise to an antiviral activity during various viral infections, such as influenza virus (29), rhinovirus (2), and human immunodeficiency virus infections (5, 32).
In consideration of the potent induction of HSP by GGA, we investigated whether oral administration (similar to clinical usage) of GGA can induce protective effects against influenza virus in vivo and we examined its possible mechanisms in vitro. This is a completely different concept from those of previous treatments, which have concentrated on immunization with the viral factor alone, in that it directly influences innate host factors prior to infection. We are confident that our findings have the potential to lead to a totally new way of treating influenza virus infection.
MATERIALS AND METHODS
Reagent and GGA treatment.
GGA was a gift from Eisai Co. (Tokyo, Japan). For oral administration to mice, a pure GGA solution supplemented with 0.2% α-tocopherol was diluted with 5% gum arabic in 100 μl; a 5% gum arabic solution containing 0.008% α-tocopherol (vehicle) was given to control mice. For treatment of cells grown in cultures, GGA supplemented with α-tocopherol was dissolved in absolute ethanol (final concentration, <0.1%). Control cells were treated with GGA-free α-tocopherol as the vehicle. Cells were treated with GGA or vehicle in serum-starved minimal Eagle’s medium (MEM)-1% fetal calf serum (FCS) for 60 min.
Virus and cells.
Influenza virus strain A/PR8/34 (H1N1) was grown for 48 h at 35 to 36°C in the allantoic cavity of 10-day-old embryonated chicken eggs and collected. Virus titers were determined with plaque assays. Influenza virus-sensitive A549 cells (kindly provided by K. Shimizu, Department of Microbiology, Nihon University School of Medicine, Tokyo, Japan) derived from a human alveolar epithelial cell were maintained in MEM containing 5% FCS. Madin-Darby canine kidney (MDCK) cells were purchased from the American Type Culture Collection (ATCC; Manassas, Va.) and maintained in MEM containing 10% FCS.
Infection models and clinical evaluation.
Specific-pathogen-free female 6-week-old BALB/c mice were obtained from Charles River Japan Co. Ltd. (Kanagawa, Japan). All experiments were conducted with the approval of the Oita Medical University Animal Experiments Ethical Standards Committee. To evaluate whether GGA (administered via oral dosage) might also actually have an antiviral activity in controlling influenza A virus infection in vivo, we administered vehicle or GGA to mice according to the following four dosage groups (30 mice per group) before infecting them with strain PR8: (i) vehicle (control); (ii) 150 mg of high-dose GGA (HDG)/kg of body weight; (iii) 75 mg of middle-dose GGA (MDG)/kg; and (iv) 15 mg of low-dose GGA (LDG)/kg. Mice were orally administered the indicated dose of GGA or vehicle twice daily at 12-h intervals for 3 weeks, according to a method described in a previous report (13), followed by intranasal infection with strain PR8 (2 × 105 PFU) while mice were awake by depositing 10 μl of virus suspension into the nostrils (5 μl/each nostril) (24). Mice were weighed immediately prior to the viral challenge and then on days 1, 2, 3, 4, 7, and 10 postchallenge. The viral titer in the lung was determined using the plaque assay method, which was conducted using MDCK cells. Viral loads in lung tissue were determined by 10-fold serial dilution of tissue extracts, and infectivity of MDCK cells in 24-well plates after 48 h of incubation at 37°C under 5% CO2 was tested. Virus titers were estimated according to ordinary plaque assays using MDCK cells; the threshold of virus detection in the MDCK assay was 1 (log10 of 10).
GGA treatment and in vitro infection assay.
A549 cells in MEM containing 5% FCS were grown to 80% confluence in plastic cell culture bottles (Falcon) (175 cm2) or 35-mm-diameter dishes (Falcon). After one washing with phosphate-buffered saline, cells were treated with the desired concentration of GGA or vehicle in serum-free OPTI-MEM (Gibco) for 1 h at 37°C under 5% CO2. Continuously, cells were washed with phosphate-buffered saline and inoculated with strain PR8 at a multiplicity of infection of 3. After absorption for 60 min, the inoculum was removed and the cells was maintained in MEM containing 5% FCS.
Histopathologic analysis.
At 10 days postinfection (p.i.), freshly isolated lung tissue samples from the control and HDG groups were fixed in 10% neutral buffered formalin, dehydrated, embedded in paraffin, and cut into 4-μm-thick sections. Sections were stained with hematoxylin and eosin.
Northern blotting, protein labeling with l-[35S]methionine, and Western blot analysis.
Total RNA was purified by Isogen extraction (Nippon Gene, Tokyo, Japan). Total RNA (20 μg) was separated on 1% formaldehyde agarose gels, transferred to a nylon membrane, and then hybridized with 32P-labeled human or mouse HSP70 cDNA (ATCC 57494 or 1375747, respectively), human myxovirus resistance 1 (MxA) cDNA (ATCC 61806), or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (ATCC 57090) as a cDNA probe. Mouse or human HSP cDNA probes were applied to total RNA derived from mouse (in vivo study) or A549 (in vitro study) cells, respectively. The bound probes were detected by autoradiography. In vitro A549 cells, with or without GGA treatment, were infected with strain PR8 or mock infected for 60 min of absorption in 35-mm-diameter dishes and maintained in MEM-5% FCS for the indicated times. The cells were incubated for 45 min in methionine-free RPMI 1640 supplemented with 2 μCi of l-[35S]methionine. Cells were lysed in ordinary sodium dodecyl sulfate (SDS) gel loading buffer (50 mM Tris-HCl [pH 6.8], 100 mM dithiothreitol [DTT], 2% SDS, 0.1% bromophenol blue, 10% glycerol) followed by SDS-polyacrylamide gel electrophoresis (PAGE). For immunoblotting, polyclonal antibodies specific for human HSP70 (Upstate Biotechnology, Lake Placid, N.Y.) (1:1,000 dilution), polyclonal α subunit of eukaryotic initiation factor 2 (eIF-2α) phosphoserine 51 (Upstate Biotechnology) (1:500 dilution), and anti-nucleoprotein (anti-NP) (Hytest, Turku, Finland) (monoclonal mouse anti-influenza virus type A NP) (1:50 dilution) were used as a primary antibody. After incubation with the above-named antibodies, the blotted membranes were washed and then incubated with secondary antibodies, namely, peroxidase-labeled anti-mouse (1:5,000 dilution) or anti-rabbit (1:2,000) antibodies (Cappel, Malvern, Pa.). After washing, membranes were subjected to an ECL kit WB detection system (Amersham Japan, Tokyo, Japan) and exposed to X-ray film.
RT-PCR.
Interferon-inducible double-strand RNA-activated protein kinase (PKR) gene expression was determined using reverse transcription-PCR (RT-PCR). The PCR was performed using the following primers: for PKR, sense primer 5′-GCAGTTAGTCCTT TATTATT-3′ and antisense primer 5′-TTGTTCGCTTTCCATCATTT-3′. The RT-PCR products obtained were then subjected to electrophoresis with 1% agarose gels.
Preparation of cytoplasmic extracts for in vitro kination assay.
Confluent monolayers of the A549 cell line with or without GGA were grown on 24-well cell culture plates. At the indicated time p.i., the medium was removed by aspiration and cells were lysed with a buffer containing 20 mM HEPES (pH 7.4), 120 mM KCl, 5 mM MgCl2, 1 mM DTT, 0.5% NP-40, 2 μg of leupeptin/ml and 50 μg of aprotinin/ml. After the nuclei were removed by low-speed centrifugation, cytoplasmic extracts were normalized for concentration before being used with a Bio-Rad protein microassay method. Each in vitro kination reaction mixture contained 20 μl of cell extract, 7.5 μl of reaction buffer (20 mM HEPES [pH 7.4], 120 mM KCl, 5 mM MgCl2, 1 mM DTT, 10% glycerol), and 7 μl of ATP mixture (1 μCi of [γ-32P]ATP in 7 μl of reaction buffer) and was incubated for 15 min at 37°C. Immediately after incubation, the labeled extracts were boiled in Laemmli-SDS sample buffer and then separated by SDS-PAGE and autoradiography at −70°C overnight.
Statistical analysis.
Differences between groups were first examined by analysis of variance. Data showing significant differences were further analyzed by Fisher's protected least significant difference (PLSD). All data are expressed as means ± standard errors of the means (SEM). A P value of less than 0.05 denoted the presence of a statistically significant difference.
RESULTS
GGA has antiviral effects during strain PR8 infection in vivo.
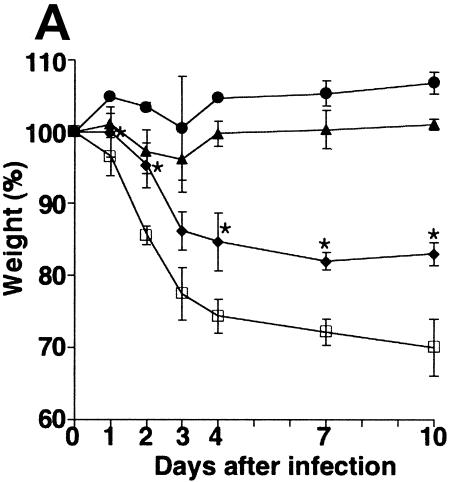
At 10 days p.i., the body weights of the control mice had dropped to 70% of their original weights (Fig. 1A). The control group displayed extremely serious symptoms of the infection from day 2 p.i., with piloerection, shivering, cessation of feeding and drinking, decreased activity, and a marked reduction in spontaneous movement as well as reduced reactivity to stimuli. In contrast, the groups that had been pretreated with GGA demonstrated dramatically dose-dependent suppression of weight loss. In particular, HDG and MDG mice exhibited continuous weight increase after 3 days p.i.
FIG. 1.
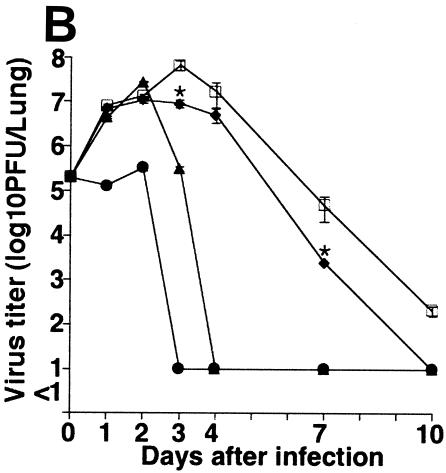
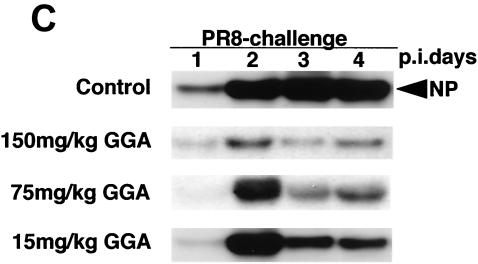
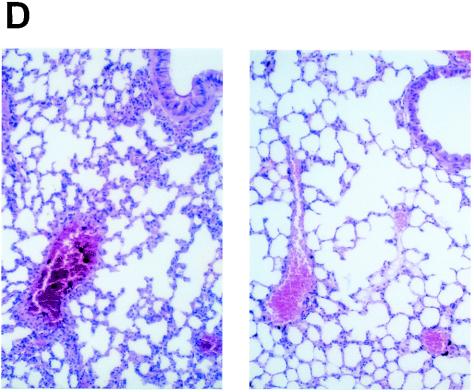
Effect of GGA administration on strain PR8-infected mice. (A) Effect of GGA on weight loss. Mice were divided into four groups with vehicle or GGA administered as follows: (i) vehicle (control group) (open squares; n = 30); (ii) 150 mg of GGA/kg of body weight (closed circles; n = 30); (iii) 75 mg of GGA/kg (closed triangles; n = 30); and (iv) 15 mg of GGA/kg (closed diamonds; n = 30). We then inoculated these mice intranasally with influenza A virus (A/PR8/34 [H1N1]) and monitored them for 10 days. Each bar represents the mean ± SEM of the results for one group (Fisher's PLSD; *, P < 0.05 versus control). (B) Viral titers in mouse lung tissue determined with a plaque assay. Titers were determined via serial dilutions of lung homogenates onto MDCK monolayer cells for detection of PFU. The results are a combination of those obtained in three separate experiments. Each bar represents the mean ± SEM of the results for one group (Fisher's PLSD; *, P < 0.01 versus control). (C) Western blot analysis of viral NP synthesis in lung tissues at an early stage. (D) Histopathologic features of the lungs of PR8-infected mice treated with or without GGA at 10 days p.i. Left panel, results for vehicle-treated control; right panel, results from treatment with 150 mg of GGA/kg. (Hematoxylin and eosin staining was used; original magnification, ×200.)
We then examined the virus titers in the lungs to evaluate virus replication in these mice. Amazingly, GGA pretreatment of mice led to a sudden deletion of strain PR8 at an early stage (Fig. 1B). In HDG mice, intrapulmonary viruses (loaded at 2 × 105 PFU) had disappeared completely without any notable replication within 3 days. The MDG and LDG groups showed similar tendencies to that of the HDG group but required a longer time period for the virus to be cleared completely. However, the duration was still apparently shorter than for ordinary PR8 infection. These results confirmed the suppression of viral propagation by GGA in a dose-dependent manner. Furthermore, we measured the amount of NP synthesis within lung protein at the early stage. The control mouse group showed consistently increased NP synthesis compared with the GGA-treated groups, which were observed to exhibit suppression of NP synthesis in a dose-dependent manner (Fig. 1C). Histologic examination of the lungs of control mice showed marked atelectasis, interstitial hypertrophy, and infiltration of neutrophils similar to the effects seen with acute lung injury (Fig. 1D, left panel). In contrast, mice of the HDG group demonstrated relatively normal architecture, with occasional infiltration of inflammatory cells in the lungs (Fig. 1D, right panel); hence, GGA resulted in inhibition of viral replication in the PR8-infected model in vivo.
GGA facilitates HSP70 synthesis in the absence or presence of infection in vivo.
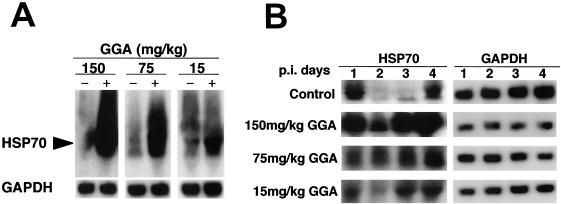
Oral administration of GGA induces HSP70 into various organs such as the stomach (13), liver (41), heart (25), and small intestine (39). Hence, we examined whether oral administration of GGA might induce HSP70 in lungs; results demonstrated that GGA has the potential to induce HSP70 mRNA signals in mouse lungs in a dose-dependent response (Fig. 2A). No HSP70 mRNA gene was detected in the control group. We verified that there were no particular adverse effects (such as weight loss, shivering, hair loss, diarrhea, reduced number of spontaneous movements, and hypothermia) and found that weight gain was unaffected, even in the HDG group, during the 3-week period of GGA administration (data not shown). When GGA was administered for less than 3 weeks (i.e., for 1, 3, or 5 days or 1 or 2 weeks), no HSP70 mRNA expression was detected in lungs, unlike the results seen with other organs (data not shown). Furthermore, we confirmed preliminary to this study that the GGA antiviral effect required at least 3 weeks of drug administration. During the early stage of strain PR8 infection, control mice showed markedly suppressed HSP70 gene expression, especially at 2 and 3 days p.i., which was presumed to be a shutoff phenomenon exhibited by host cells acutely infected with PR8 (Fig. 2B). Results also demonstrated that just after PR8 infection in GGA-treated groups, the HSP70 gene was highly transcribed and preserved in a dose-dependent manner. The level of transcription was prominently interrelated with weight loss and viral replication.
FIG. 2.
Expression of HSP70 mRNA induced by GGA in mouse lungs. Total RNA was extracted from the lungs of three mice in each group and analyzed by Northern blotting. (A) Results of 3 weeks of oral administration of the indicated dose of GGA or of the corresponding dose of vehicle in the uninfected mice. (B) HSP70 mRNA expression with strain PR8 after GGA administration on the indicated day p.i. Left panel, HSP70 (lanes 1, 2, 3 and 4 correspond to days 1, 2, 3, and 4, respectively); right panel, GAPDH.
GGA induces HSP70 in vitro.
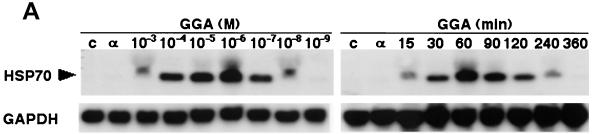
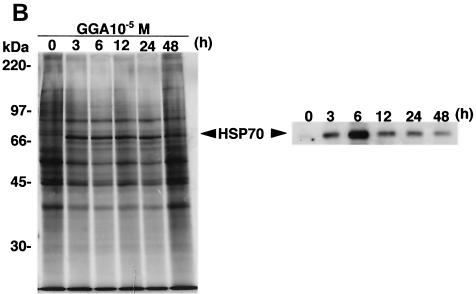
GGA treatment of A549 cells derived from a human alveolar epithelial cell line resulted in apparent HSP70 gene transcription in a both dose- and time-dependent manner (Fig. 3A). Similarly, we detected that GGA treatment increased HSP70 synthesis and accumulation, showing peak levels at 6 h after treatment and remaining for 48 h (Fig. 3B). From these results we determined by in vitro assays that for further analysis, the optimal conditions for treatment of A549 cells were 10−5 M GGA for 60 min.
FIG. 3.
Induction of HSP70 expression by GGA treatment in vitro. Cells of the A549 human alveolar epithelial cell line were treated with GGA at serial concentrations and times in MEM-1% FCS. (A) HSP70 transcripts formed in a dose- and time-dependent manner. (B) Kinetics of HSP70 synthesis and accumulation under optimal conditions. A549 cells were treated with GGA at 10−5 M for 60 min in MEM-1% FCS and were maintained for the indicated times in MEM-5% FCS. Concurrently, the cells were labeled with [35S]methionine as described in Material and Methods. Samples containing equal amounts of radioactivity were subjected to SDS-PAGE (left panel) and immunoblot analysis with anti-HSP70 antibody (right panel).
GGA suppresses virus-associated protein synthesis and accumulation.
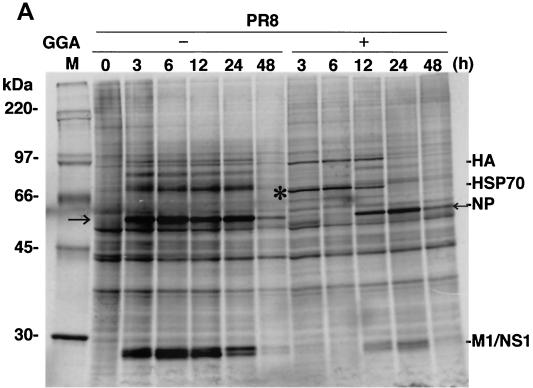
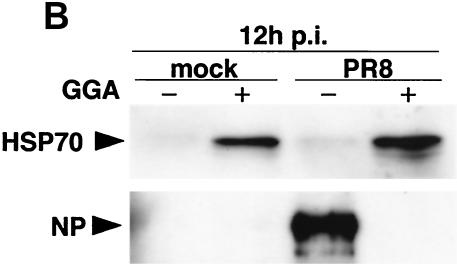
Infection of control cells with strain PR8 resulted in elevated kinetics of de novo NP synthesis as well as elevated kinetics of other virus-associated proteins, such as HA, M1, and NS1 (Fig. 4A). At 48 h p.i., more than 80% of cells exhibited a significant cytopathic effect. In contrast, PR8-infected cells treated with GGA showed a complete block of NP synthesis corresponding to augmentation of HSP70 at an early stage (within 6 h). The delayed NP synthesis was initiated gradually with simultaneous diminution of HSP70 synthesis from 12 h p.i.; however, the level of NP had obviously decreased. The antiviral action produced by GGA is more prominently revealed with immunoblot analysis (Fig. 4B). At least 12 h p.i., NP accumulation during PR8 infection was not detectable in GGA-pretreated cells compared to that seen with untreated cells. The defect in virus protein synthesis correlated well with the level of HSP70.
FIG. 4.
Effect of GGA on viral protein synthesis in vitro. (A) Kinetics of strain PR8-associated proteins and HSP70 synthesis in PR8-infected A549 cells with GGA treatment (10−5 M for 60 min) or without GGA treatment. After the 1-h PR8 adsorption period, the cells were maintained for the indicated times. Cells labeled with [35S]methionine were lysed and subjected to SDS-PAGE. The asterisk and the arrows indicate HSP70 and NP, respectively. (B) Accumulated levels of HSP70 and viral NP at 12 h p.i. with PR8. Samples obtained at 12 h in the above-described experiment were subjected to immunoblot analysis with anti-HSP70 and anti-NP antibodies. Mock, mock infected.
GGA-stimulated antiviral factors during PR8 infection.
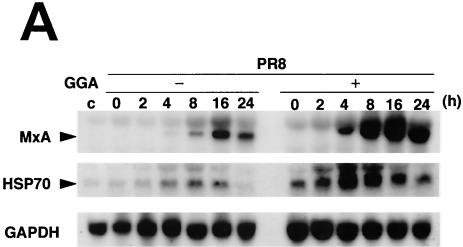
MxA protein (encoded by chromosome 21q) belongs to the dynamin superfamily of large GTPases, for which GTPase activity is a prerequisite for antiviral activity, particularly that of members of the family Myxoviridae (18). Then, MxA protein appears to block the replication of certain viruses. Up-regulation of MxA gene transcription induced in infected cells treated with GGA proceeded more rapidly and more strongly than that seen in infected cells without GGA treatment (Fig. 5A). Furthermore, up-regulation of MxA by GGA synchronized with an increase of HSP70 expression in GGA-treated cells which was sustained for a period of at least 48 h.
FIG. 5.
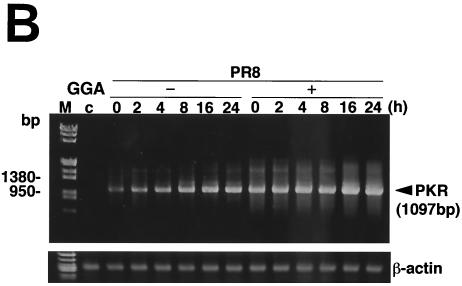
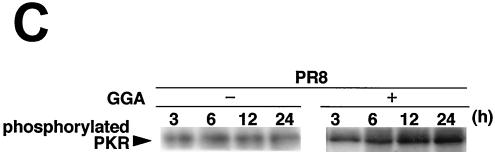
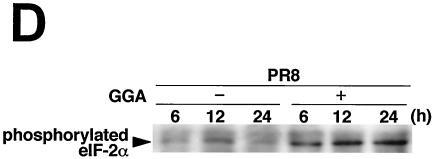
GGA effects on antiviral factors during strain PR8 infection. A549 cells were treated with or without GGA followed by PR8 infection and incubated for the time period indicated. (A) mRNA expression of the antiviral gene (MxA), HSP70, and GAPDH. (B) Enhanced PKR mRNA expression by GGA, as analyzed by RT-PCR assay. (C) Level of PKR autophosphorylation. (D) Determination (using immunoblot analysis) of levels of phosphorylated eIF-2α, which is activated by phosphorylated PKR.
Finally, to elucidate the direct antiviral effects of GGA, we analyzed double-strand PKR. PKR is a part of the interferon-inducible, well-studied mediator of host cellular defense against virus infection (33, 36). GGA treatment apparently enhanced the level of PKR gene transcription in PR8-infected cells compared to that seen without GGA treatment and, as a consequence, sustained the level and duration of the PKR effect (Fig. 5B). Phosphorylated PKR, which has been characterized most extensively as a host defense against viral infection, appeared more rapidly at 3 h p.i. (Fig. 5C). Furthermore, we determined the level of eIF-2α at the phosphorylation level by immunoblotting, because phosphorylated PKR sequentially phosphorylates eIF-2α (8) and leads to inhibition of the translation initiation of the host cell as well as that of virus, thus contributing to the interferon-mediated defense mechanism for restricting viral protein translation, ultimately resulting in the inhibition of viral replication (34, 37). As a consequence of the promotion of PKR phosphorylation, GGA (as expected) facilitated phosphorylation of eIF-2α (Fig. 5D). Neither PKR nor eIF-2α phosphorylation was detected in cells not infected with PR8 (data not shown). From these results, GGA was confirmed to be implicated in activation of these antiviral factors.
DISCUSSION
In the present study, we raised the possibility of a new treatment for prevention of influenza A virus infection. To date, inactivated hemagglutinin vaccine has been widely used for members of older age groups and has shown a degree of protective efficacy. However, the emergence of mutant strains of influenza A virus frequently appears to result from antigen shifts due to RNA segment genetic reassortment or antigenic drift due to RNA mutation, which makes complete prevention impossible. In such situations, humans cannot help but rely on intrinsic antiviral mechanisms. Innate human reactions combating influenza virus are almost always nonspecific, especially during the acute phase. Fever is the most typical response against viral infection, and HSPs are generally induced by fever. If it is possible to induce effective HSPs without any harmful effects, such inducement is desirable as a blockade against various infectious pathogens, especially viruses. In fact, it has been reported that HSP70 induction by cyclopentenone prostanoids led to antiviral activity in the broad-spectrum DNA and RNA virus infection models (35). We hypothesized that GGA can induce antiviral proteins directly or indirectly via the introduction of abundant HSP70 in lungs, resulting in impairment of the virus replication activity (Fig. 1 and 2).
As shown in Fig. 4A, newly synthesized viral proteins (NP and NS1 of strain PR8) were markedly inhibited by GGA treatment. This viral protein synthesis might correlate closely with the kinetics of HSP70. From this result, we speculated that the essential GGA mechanism during PR8 infection in vivo and in vitro was the induction of HSPs. HSP70 is a crucial molecular chaperone for the maintenance of cell integrity during growth and homeostasis and is considered to exert normalization on intracellular targets and inhibition on viral targets at various stages in the process of viral replication. Antiviral activity against poliovirus replication disappears in cells in which a lack of an ability to synthesize HSP70 or shutoff of HSP70 synthesis in the infected cells during rotavirus infection is noted (3, 38). However, little is yet known concerning the detailed mechanism by which HSP70 interferes with viral replication. In the present study, we found by several analyses in vitro that one of the roles of HSP70 is that of a potent activator of antiviral genes.
A key component of interferon-mediated action against viruses is the human MxA protein. In in vitro analyses, influenza A virus-infected A549 cells also produced distinct MxA gene expression, as reported previously (30). MxA exhibits antiviral activity against various RNA viruses, including influenza C virus (22) as well as type A (28). MxA acts by blocking the transport of viral nucleocapsids into the nucleus of the host cells, resulting in the failure of the viral genome to replicate (18). During strain PR8 infection, GGA induced up-regulation of cellular MxA gene expression to higher levels and more rapidly than that seen with the control, synchronizing augmentation of the HSP70 gene. Impaired virus replication, as observed by in vitro studies, may be explained by increased MxA transcription, and GGA-induced HSP70 may promote the accumulation of MxA protein in the cytoplasm of host cells. The transgenic mice permanently expressing human MxA protein appeared resistant to influenza virus infection (27); therefore, we presumed that the beneficial effects of in vivo experiments might be derived from up-regulation of the MxA gene by GGA.
The interferon-inducible protein kinase PKR is a well-characterized effector against extensive viral infection. Activation of PKR resulted in autophosphorylation and subsequent phosphorylation of eIF-2α, which inhibited translation initiation of viral proteins. The decline in protein synthesis causes virus replication to rapidly deteriorate; however, various viruses circumvent this PKR function (17, 19). For example, influenza virus encodes a gene product which functions to directly block the autophosphorylation and activity of PKR (16). Through this function, influenza virus avoids a variety of host defense responses. GGA treatment prominently elevated the level of PKR gene transcription in strain PR8-infected cells and, as a consequence, sustained the level and duration of PKR autophosphorylation, whereas both were attenuated in control cells. These data indicate that GGA enhances interferon-mediated antiviral action not only by induction of MxA gene expression but also by another cascade, such as PKR activation, resulting from a possible unknown mechanism in synergy. The promoted eIF-2α phosphorylation consolidated the antiviral mechanism of GGA. On the other hand, GGA treatment abolished viral protein synthesis, including that of NS1 and NP proteins (Fig. 4A). These viral proteins are major components of influenza virus propagated in infected cells, and it is easy to determine whether viral propagation increases or decreases. The NS1 is a unique protein that is indispensable for successful influenza virus infection. This protein efficiently eliminated PKR activation by binding to the double-strand RNA activator (21); hence, mutant influenza virus with defective NS1 protein cannot suppress the autophosphorylation of PKR during virus growth, resulting in elevation of the level of phosphorylation of eIF-2α (11). It is possible that the intact phosphorylation of eIF-2α, as well as that of PKR, produced by GGA contributes to the dramatic antiviral effect.
In the present study, we used GGA, which is considered an antiviral drug. HSP induction is deeply involved in the suppression of the propagation of a number of viruses other than the influenza virus (29), including rhinovirus (2), rotavirus (28), poliovirus (3), vesicular stomatitis virus (31), Sindbis virus (23), and Sendai virus (1); HSP induction is also deeply involved in the suppression of the propagation of human T-cell leukemia virus type 1 (6) and human immunodeficiency virus type 1 (5), which cause serious clinical disease. When used to induce HSP70 and following antiviral gene expression against the above-mentioned viruses, a universal antiviral effect can be exhibited. Concerning the action of HSP70, one proposition advocated that the mechanism might work by interfering in viral protein synthesis by acting directly on nascent viral polypeptides, giving rise to a translational block, or, alternatively, that it might possess a translational mechanism similar to that of the virus messages and might compete at that stage (29).
The advantages of GGA are that it is already in clinical use and has a strong track record and a proven safety profile. Laboratory data reveal no detrimental events, even at the massive dosage of 500 mg/kg of body weight (data not shown), and none were seen with the HDG group in the present study. The method of application is simple, with induction of HSP70 expression achieved with oral dosages. If HSP70 affords protection against a variety of viral infections, as has been reported previously, it should be usable as a universal agent with no need to choose the target virus antigen; the problem of antigenic change would then disappear. Interesting evidence has been reported indicating that GGA expressed the antiviral genes 2′5′-oligoadenylate synthetase (2′5′-OAS) and PKR through the formation of interferon-stimulated gene factor 3 and that GGA induced expression of signal transducers and activators of transcription 1 (STAT1) and STAT2 and p48 protein in hepatoma cells (14). Accordingly, we speculate that the GGA-mediated antiviral effects seen in our study may be induced by these complicated cascades. However, further studies are needed (with closely coordinated in vivo and in vitro components) to elucidate the detailed mechanism of action of GGA and the treatment method, including the dose or the phase, before GGA treatment can be used reliably.
Acknowledgments
We express our gratitude to K. Shimizu for providing technical advice as well as the A549 cells, K. Rokutan for helpful discussion, and Eisai Co. for generously providing the GGA.
REFERENCES
- 1.Amici, C., C. Giorgi, A. Rossi, and M. G. Santoro. 1994. Selective inhibition of virus protein synthesis by prostaglandin A1: a translational block associated with HSP70 synthesis. J. Virol. 68:6890-6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conti, C., A. De Marco, P. Mastromarino, P. Tomao, and M. G. Santoro. 1999. Antiviral effect of hyperthermic treatment in rhinovirus infection. Antimicrob. Agents Chemother. 43:822-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conti, C., P. Mastromarino, P. Tomao, A. De Marco, F. Pica, and M. G. Santoro. 1996. Inhibition of poliovirus replication by prostaglandins A and J in human cells. Antimicrob. Agents Chemother. 40:367-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couch, R. B., J. A. Kasel, W. P. Glezen, T. R. Cate, H. R. Six, L. H. Taber, A. L. Frank, S. B. Greenberg, J. M. Zahradnik, and W. A. Keitel. 1986. Influenza: its control in persons and populations. J. Infect. Dis. 153:431-440. [DOI] [PubMed] [Google Scholar]
- 5.De Marco, A., A. Carattoli, C. Rozera, D. Fortini, C. Giorgi, G. Belardo, C. Amici, and M. G. Santoro. 1998. Induction of the heat-shock response by antiviral prostaglandins in human cells infected with human immunodeficiency virus type 1. Eur. J. Biochem. 256:334-341. [DOI] [PubMed] [Google Scholar]
- 6.D'Onofrio, C., O. Franzese, A. De Marco, E. Bonmassar, and C. Amici. 1994. Antiproliferative activity of cyclopentenone prostaglandins in early HTLV-1 infection is independent of IL-2 and is associated with HSP70 induction. Leukemia 8:1045-1056. [PubMed] [Google Scholar]
- 7.Englund, J. A., R. E. Champlin, P. R. Wyde, H. Kantarjian, R. L. Atmar, J. Tarrand, H. Yousuf, H. Regnery, A. I. Klimov, N. J. Cox, and E. Whimbey. 1998. Common emergence of amantadine- and rimantadine-resistant influenza A viruses in symptomatic immunocompromised adults. Clin. Infect. Dis. 26:1418-1424. [DOI] [PubMed] [Google Scholar]
- 8.Galabru, J., and A. Hovanessian. 1987. Autophosphorylation of the protein kinase dependent on double-stranded RNA. J. Biol. Chem. 262:15538-15544. [PubMed] [Google Scholar]
- 9.Glezen, W. P. 1982. Serious morbidity and mortality associated with influenza epidemics. Epidemiol. Rev. 4:25-44. [DOI] [PubMed] [Google Scholar]
- 10.Gubareva, L. V., L. Kaiser, and F. G. Hayden. 2000. Influenza virus neuraminidase inhibitors. Lancet 355:827-835. [DOI] [PubMed] [Google Scholar]
- 11.Hatada, E., S. Saito, and R. Fukuda. 1999. Mutant influenza viruses with a defective NS1 protein cannot block the activation of PKR in infected cells. J. Virol. 73:2425-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayden, F. G., A. D. Osterhaus, J. J. Treanor, D. M. Fleming, F. Y. Aoki, K. G. Nicholson, A. M. Bohnen, H. M. Hirst, O. Keene, and K. Wightman. 1997. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. N. Engl. J. Med. 337:874-880. [DOI] [PubMed] [Google Scholar]
- 13.Hirakawa, T., K. Rokutan, T. Nikawa, and K. Kishi. 1996. Geranylgeranylacetone induces heat shock proteins in cultured guinea pig gastric mucosal cells and rat gastric mucosa. Gastroenterology 111:345-357. [DOI] [PubMed] [Google Scholar]
- 14.Ichikawa, T., K. Nakao, K. Nakata, K. Hamasaki, Y. Takeda, Y. Kajiya, S. Higashi, K. Ohkubo, Y. Kato, N. Ishii, and K. Eguchi. 2001. Geranylgeranylacetone induces antiviral gene expression in human hepatoma cells. Biochem. Biophys. Res. Commun. 280:933-939. [DOI] [PubMed] [Google Scholar]
- 15.Jefferson, T. O., V. Demicheli, J. J. Deeks, and D. Rivetti. 2001. Amantadine and rimantadine for preventing and treating influenza A in adults. Cochrane Database Syst. Rev. 2:CD001169. [Online.] [DOI] [PubMed]
- 16.Katze, M. G., J. Tomita, T. Black, R. M. Krug, B. Safer, and A. Hovanessian. 1988. Influenza virus regulates protein synthesis during infection by repressing autophosphorylation and activity of the cellular 68,000-Mr protein kinase. J. Virol. 62:3710-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitajewski, J., R. J. Schneider, B. Safer, S. M. Munemitsu, C. E. Samuel, B. Thimmappaya, and T. Shenk. 1986. Adenovirus VAI RNA antagonizes the antiviral action of interferon by preventing activation of the interferon-induced eIF-2 α kinase. Cell 45:195-200. [DOI] [PubMed] [Google Scholar]
- 18.Kochs, G., and O. Haller. 1999. Interferon-induced human MxA GTPase blocks nuclear import of Thogoto virus nucleocapsids. Proc. Natl. Acad. Sci. USA 96:2082-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, T. G., J. Tomita, A. G. Hovanessian, and M. G. Katze. 1990. Purification and partial characterization of a cellular inhibitor of the interferon-induced protein kinase of Mr 68,000 from influenza virus-infected cells. Proc. Natl. Acad. Sci. USA 87:6208-6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long, J. K., S. B. Mossad, and M. P. Goldman. 2000. Antiviral agents for treating influenza. Clevel. Clin. J. Med. 67:92-95. [DOI] [PubMed] [Google Scholar]
- 21.Lu, Y., M. Wambach, M. G. Katze, and R. M. Krug. 1995. Binding of the influenza virus NS1 protein to double-stranded RNA inhibits the activation of the protein kinase that phosphorylates the eIF-2 translation initiation factor. Virology 214:222-228. [DOI] [PubMed] [Google Scholar]
- 22.Marschall, M., A. Zach, A. Hechtfischer, G. Foerst, H. Meier-Ewert, and O. Haller. 2000. Inhibition of influenza C viruses by human MxA protein. Virus Res. 67:179-188. [DOI] [PubMed] [Google Scholar]
- 23.Mastromarino, P., C. Conti, R. Petruzziello, A. De Marco, F. Pica, and M. G. Santoro. 1993. Inhibition of Sindbis virus replication by cyclopentenone prostaglandins: a cell-mediated event associated with heat-shock protein synthesis. Antivir. Res. 20:209-222. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen, H. H., Z. Moldoveanu, M. J. Novak, F. W. van Ginkel, E. Ban, H. Kiyono, J. R. McGhee, and J. Mestecky. 1999. Heterosubtypic immunity to lethal influenza A virus infection is associated with virus-specific CD8+ cytotoxic T lymphocyte responses induced in mucosa-associated tissues. Virology 254:50-60. [DOI] [PubMed] [Google Scholar]
- 25.Ooie, T., N. Takahashi, T. Saikawa, T. Nawata, M. Arikawa, K. Yamanaka, M. Hara, T. Shimada, and T. Sakata. 2001. Single oral dose of geranylgeranylacetone induces heat-shock protein 72 and renders protection against ischemia/reperfusion injury in rat heart. Circulation 104:1837-1843. [DOI] [PubMed] [Google Scholar]
- 26.Ouyang, Q., G. Cicek, R. G. Westendorp, H. J. Cools, R. J. van der Klis, and E. J. Remarque. 2000. Reduced IFN-γ production in elderly people following in vitro stimulation with influenza vaccine and endotoxin. Mech. Ageing Dev. 121:131-137. [DOI] [PubMed] [Google Scholar]
- 27.Pavlovic, J., H. A. Arzet, H. P. Hefti, M. Frese, D. Rost, B. Ernst, E. Kolb, P. Staeheli, and O. Haller. 1995. Enhanced virus resistance of transgenic mice expressing the human MxA protein. J. Virol. 69:4506-4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavlovic, J., O. Haller, and P. Staeheli. 1992. Human and mouse Mx proteins inhibit different steps of the influenza virus multiplication cycle. J. Virol. 66:2564-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pica, F., A. T. Palamara, A. Rossi, A. De Marco, C. Amici, and M. G. Santoro. 2000. Δ12-prostaglandin J2 is a potent inhibitor of influenza A virus replication. Antimicrob. Agents Chemother. 44:200-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ronni, T., S. Matikainen, T. Sareneva, K. Melén, J. Pirhonen, P. Keskinen, and I. Julkunen. 1997. Regulation of IFN-α/β, MxA, 2′,5′-oligoadenylate synthetase, and HLA gene expression in influenza A-infected human lung epithelial cells. J. Immunol. 158:2363-2374. [PubMed] [Google Scholar]
- 31.Rossi, A., G. Elia, and M. G. Santoro. 1996. 2-Cyclopenten-1-one, a new inducer of heat shock protein 70 with antiviral activity. J. Biol. Chem. 271:32192-32196. [DOI] [PubMed] [Google Scholar]
- 32.Rozera, C., A. Carattoli, A. De Marco, C. Amici, C. Giorgi, and M. G. Santoro. 1996. Inhibition of HIV-1 replication by cyclopentenone prostaglandins in acutely infected human cells. J. Clin. Investig. 97:1795-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samuel, C. E. 1991. Antiviral actions of interferon. Interferon-regulated cellular proteins and their surprisingly selective antiviral activities. Virology 183:1-11. [DOI] [PubMed] [Google Scholar]
- 34.Samuel, C. E. 1993. The eIF-2α protein kinases, regulators of translation in eukaryotes from yeasts to humans. J. Biol. Chem. 268:7603-7606. [PubMed] [Google Scholar]
- 35.Santoro, M. G. 1997. Antiviral activity of cyclopentenone prostanoids. Trends Microbiol. 5:276-281. [DOI] [PubMed] [Google Scholar]
- 36.Sen, G. C., and P. Lengyel. 1992. The interferon system. A bird's eye view of its biochemistry. J. Biol. Chem. 267:5017-5020. [PubMed] [Google Scholar]
- 37.Staeheli, P. 1990. Interferon-induced proteins and the antiviral state. Adv. Virus Res. 38:147-200. [DOI] [PubMed] [Google Scholar]
- 38.Superti, F., C. Amici, A. Tinari, G. Donelli, and M. G. Santoro. 1998. Inhibition of rotavirus replication by prostaglandin A: evidence for a block of virus maturation. J. Infect. Dis. 178:564-568. [DOI] [PubMed] [Google Scholar]
- 39.Tsuruma, T., A. Yagihashi, S. Koide, J. Araya, K. Tarumi, N. Watanabe, and K. Hirata. 1999. Geranylgeranylacetone induces heat shock protein-73 in rat small intestine. Transplant. Proc. 31:572-573. [DOI] [PubMed] [Google Scholar]
- 40.Wingfield, W. L., D. Pollack, and R. R. Grunert. 1969. Therapeutic efficacy of amantadine HCl and rimantadine HCl in naturally occurring influenza A2 respiratory illness in man. N. Engl. J. Med. 281:579-584. [DOI] [PubMed] [Google Scholar]
- 41.Yamagami, K., Y. Yamamoto, Y. Ishikawa, K. Yonezawa, S. Toyokuni, and Y. Yamaoka. 2000. Effects of geranyl-geranyl-acetone administration before heat shock preconditioning for conferring tolerance against ischemia-reperfusion injury in rat livers. J. Lab. Clin. Med. 135:465-475. [DOI] [PubMed] [Google Scholar]