Abstract
The first outbreak of multidrug-resistant (MDR) typhoid fever in Vietnam was in 1993, and by 1995 nearly 90% of cases were MDR. Plasmid HCM1, sequenced in full, is an incHI1 plasmid from Salmonella enterica serovar Typhi strain CT18, isolated in Vietnam in 1993. Restriction analysis shows that pHCM1 shares a restriction fragment length polymorphism (RFLP) pattern with plasmids isolated from the first outbreak and 10 of 17 MDR plasmids isolated from sporadic cases occurring at the same time in Vietnam. A core region of pHCM1 has significant DNA sequence similarity to plasmid R27, isolated in 1961 from S. enterica in the United Kingdom. There are five regions of DNA in pHCM1 which are not present in R27. Two of these are putative acquisition regions; the largest is 34.955 kbp in length and includes sequences of several antibiotic resistance genes and several insertion sequences. The borders of this region are defined by two identical IS10 left elements, associated with an inversion of DNA or with a truncated Tn10 element. The second, smaller region is 14.751 kbp and carries a trimethoprim resistance gene dfr14A cassette associated with a class 1 integrase. In 1993 to 1994, restriction analysis revealed some variations in the structures of Salmonella serovar Typhi MDR plasmids which were mapped to the two putative acquisition regions and three smaller variable regions. In 1996 a single RFLP type, RFLP7, was found to carry the dfrA7 and sul-1 genes, which were not present on R27 or pHCM1. This plasmid type appears to have a selective advantage over other plasmids with the same resistance phenotype.
Resistance to multiple antimicrobial agents in bacterial pathogens is an emerging global problem that is having a serious impact on the treatment of infectious diseases (1, 4). There are undoubtedly many factors associated with the emergence of resistance. An understanding of these factors is crucial if we are to limit the spread of resistance (21). A significant proportion of drug resistance in bacteria is known to be associated with the acquisition of plasmid DNA (7), but the selective pressures that favor the maintenance of resistance are not fully defined (6). Multidrug resistance (MDR) in the causal agent of typhoid fever, Salmonella enterica subsp. enterica serovar Typhi is one example of a pathogen acquiring resistance which now poses a major threat to the effective treatment of disease (25). MDR Salmonella serovar Typhi harbors a variety of plasmids, but those of the incHI1 incompatibility type appear to be particularly common in this serovar of S. enterica (8, 20). A possible explanation for their common occurrence is a transmission potential that is enhanced compared to that of drug-sensitive strains (23).
The complete DNA sequence of the genome of an MDR Salmonella serovar Typhi strain, CT18, isolated in 1993 from a typhoid patient in Vietnam, was recently reported (13). This strain harbors an incHI1 plasmid of 218 kbp, pHCM1, that encodes transferable multiple-antibiotic resistance. Sherburne et al. (17) recently reported the full sequence of another incH plasmid, R27, isolated several decades ago from S. enterica. R27 is 180 kbp in length, encodes tetracycline resistance, and is a precursor of pHCM1 (13). It seems likely that pHCM1 has acquired two distinct regions of DNA that R27 does not have, and these regions include the genes encoding MDR. The availability of the DNA sequences of pHCM1 and R27 provides a unique opportunity to investigate the structure of incHI1 plasmid populations from Salmonella serovar Typhi. Thus, we analyzed the genetic variation in pHCM1-related incHI1 MDR plasmids isolated from patients with MDR typhoid fever in Vietnam between 1993 and 1996. This analysis enabled us to document variations in plasmid structure for pathogens from a site where a disease is endemic and antibiotics are freely available.
MATERIALS AND METHODS
DNA sequence data analysis.
The sequence of pHCM1 was obtained as part of the whole genome sequencing of Salmonella serovar Typhi CT18 (13). The DNA sequence and annotation of R27 were obtained from GenBank (accession no. AF250878) (17). Sequence analysis was carried out with Artemis and the comparison tool available from the website of the Sanger Institute, Hinxton, Cambridge, United Kingdom.
Bacterial strains and susceptibility testing.
All isolates of Salmonella serovar Typhi were from blood and bone marrow cultures from patients admitted to the Centre for Tropical Diseases (CTD), Ho Chi Minh City, Vietnam. The CTD is an admission and referral hospital for patients from Ho Chi Minh City and the southern provinces of Vietnam with acute infectious diseases. Isolates were cultured on sheep blood or nutrient agar (Oxoid, Basingstoke, United Kingdom) and stored on Protect beads (Prolabs, Oxford, United Kingdom) at −18°C. Bacterial identification was carried out at the time of isolation by agglutination with specific antisera (Murex, Dartford, United Kingdom). Identification was confirmed by biochemical testing on the following media: Kligler iron agar slants, urea slopes, Simmons citrate agar, SIM medium for motility, H2S production medium, and indole (all from Oxoid). Information on the background levels of resistance for Salmonella serovar Typhi was taken from the hospital's laboratory records of blood culture isolates. Determination of the MIC was done by the agar dilution method (12). The following powders were used: ampicillin, trimethoprim, sulfamethoxazole, chloramphenicol, tetracycline (all from Sigma, Poole, Dorset, United Kingdom), and mercuric chloride (Difco, Detroit, Mich.).
Plasmid analysis.
The first outbreak of MDR typhoid fever in Vietnam was in 1993 (22). For plasmid analysis, 30 isolates collected around this time, between December 1992 and June 1994, were selected; 24 were MDR isolates (resistant to ampicillin, chloramphenicol, trimethoprim, and sulfonamide), and 6 were fully susceptible isolates (susceptible to ampicillin, chloramphenicol, trimethoprim, and sulfamethoxazole). To look for changes related to the stability of a single MDR plasmid in the bacterial population, these isolates were matched by resistance pattern and as far as possible by home location of the patient with 30 isolates from 1996 and 3 isolates from each of four outbreaks (5). For size determination, plasmid DNA was extracted from 4.5 ml of overnight Salmonella serovar Typhi culture according to the method of Kado and Liu (9). The weight of each plasmid was calculated by comparison with that of Escherichia coli 39R standard plasmid DNA (gift from Tony Hart, Liverpool University, United Kingdom) after agarose gel electrophoresis. For restriction enzyme analysis, the DNA was precipitated with 3 M potassium acetate and absolute alcohol and washed with 1 ml of 70% ethanol. Restriction enzyme analysis was performed immediately with HindIII (Boehringer Mannheim).
Bacterial conjugation.
Bacterial conjugation was carried out by inoculating 10 ml of brain heart infusion broth (Oxoid) with approximately 106 donor Salmonella serovar Typhi organisms and an equal number of recipient nalidixic acid-resistant or streptomycin-resistant E. coli K-12 lac+ organisms. After overnight incubation at 37°C, transconjugates were selected by plating cells onto Mueller-Hinton agar containing nalidixic acid (32 μg/ml) or streptomycin (32 μg/liter), with antibiotic disks containing ampicillin, tetracycline, sulfamethoxazole, trimethoprim, and chloramphenicol being placed on the surface of the agar. The identities of putative transconjugates growing around each disk were confirmed by lactose fermentation and failure to agglutinate O9- or Vi-specific antisera. Antimicrobial susceptibility was tested by disk diffusion and agar dilution.
PCR identification of resistance genes and class 1 integrons.
Whole-cell genomic DNA was extracted from 3-ml overnight cultures of Salmonella serovar Typhi by the cetyltrimethylammonium bromide method (3). The DNA was resuspended in sterile, distilled water and stored at −20°C until use. Primers designed against the antimicrobial resistance genes cat type 1, sul-2, dfrA14, and tetA and the gene for TEM β-lactamase were used to amplify Salmonella serovar Typhi DNA by PCR (Table 1). Primers were used at a final concentration of 1 μM in a 50-ml reaction mixture containing 1.5 mM MgCl2; 200 μM (each) dATP, dCTP, dGTP, and dTTP; 1× reaction buffer (Sigma); 2 U of DNA polymerase (Sigma); and 1 μl of DNA as the template. PCR amplification was conducted in a PTC-200 peptide thermal cycler (MJ Research) by using the following program: an initial denaturation at 95.0°C for 5 min; 28 cycles of 95.0°C for 30 s, 57.5° for 1 min, and 72.0°C for 1 min with a final cycle of 95.0°C for 30 s and 57.5°C for 1 min; and a final extension of 72.0°C for 2 min. To describe the incompatibility group and to look for sulfonamide and trimethoprim resistance genes not found by using the first multiplex PCR, further primers were designed and used with the same conditions (Table 1). A selection of PCR products was sequenced to ensure that the product was specific.
TABLE 1.
Oligonucleotides for identification of resistance genes
| Primera | Sequence (5′ to 3′)b | Tmc | Length of product (bp) | Reference sequence |
|---|---|---|---|---|
| CAT-F | TCCCAATGGCATCGTAAAGAAC | 58.4 | 293 | Transposon Tn9 from E. coli (GenBank entry J01841) |
| CAT-R | TCGTGGTATTCACTCCAGAGCG | 62.1 | ||
| DHFR-F2 | TTTGATGTCCAACCTGAGCGGG | 60.6 | 189 | E. coli dhfr1b (dfrA14) gene (GenBank entry Z50804) |
| DHFR-R2 | TGCGAAAGCGAAAAACGGCG | 62.7 | ||
| DHFR7-F | GTGTCGAGGAAAGGAATTTCAAGCTC | 59.6 | 191 | E. coli dhfrVII gene Tn5086 (GenBank entry 43090) |
| DHFR7-R | TCACCTTCAACCTCAACGTGAACAG | 59.1 | ||
| DPS-F | TCAAGGCAGATGGCATTCCC | 59.4 | 156 | suII gene from Pasteurella multicoda plasmid pIGI (GenBank entry U57647.1) |
| DPS-R | CGACGAGTTTGGCAGATGATTTC | 60.6 | ||
| DPS1-F | GGATGGGATTTTTCTTGAGCCCCGC | 66.8 | 308 | Transposon Tn21 from E. coli (GenBank entry AF071413) |
| DPS1-R | ATCTAACCCTCGGTCTCTGGCGTCG | 64.3 | ||
| incH-F | CGAAATCGGTCCAACCCATTG | 58.6 | 110 | repHI1A from R27 (EMBL entry M95772) |
| incH-R | CGACAACTCATCAGAAGCGTCAAC | 57.6 | ||
| TEM-Fc | TTTTCGTGTCGCCCTTATTCC | 57.9 | 798 | General β-lactamase (TEM-1) Neisseria meningitidis plasmid pAB6 (GenBank entry AF126482) |
| TEM-R | CGTTCATCCATAGTTGCCTGACTC | 62.7 | ||
| TET-F | GCACTTGTCTCCTGTTTACTCCCC | 64.4 | 687 | Shigella flexneri transposon Tn10 (GenBank entry AF162223.1) |
| TET-R | CCTTGTGGTTATGTTTTGGTTCCG | 61.0 |
F, forward primer; R, reverse primer.
The reference sequences were assigned by using Entrez Nucleotide at NCBI (http://www.ncbi.nlm.nih.gov/Entrez/). The “T” in bold is a C in the E. coli TEM-1D β-lactamase.
Tm, melting temperature.
PCR primers specific for the integrase intI1 (M73819; Pseudomonas aeruginosa plasmid pVS1) were used to screen for the presence of class 1 integrons in each plasmid RFLP type. A primer specific for the aroC gene of Salmonella serovar Typhi was used as a control in each PCR. Primers were used at a 1 μM final concentration in a reaction volume of 25 μl containing 200 μM (each) dATP, dGTP, dCTP, and dTTP; 1× reaction buffer; 1.25 U of AmpliTaq (Applied Biosystems); and 1 μl of DNA as the template. PCR amplification was carried out by using the following program: an initial cycle at 95.0°C for 5 min; 28 cycles of 95.0°C for 30 s, 57.0°C for 1 min, and 72°C for 1 min; and a final cycle of 72.0°C for 2 min.
RESULTS
Features of pHCM1 that distinguish this plasmid from the precursor, R27.
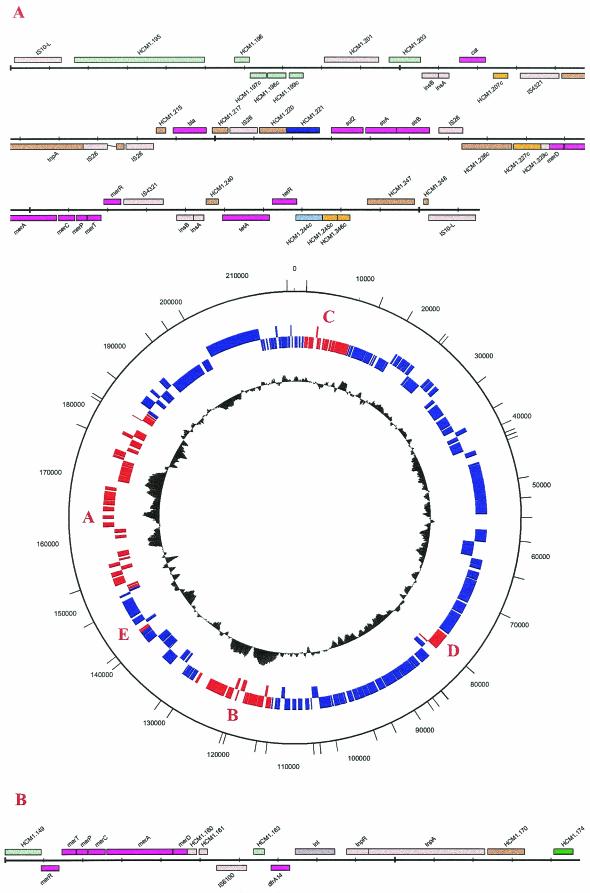
Plasmid pHCM1 is 218,160 bp in length and shares 168 kbp of DNA and >99% sequence identity with plasmid R27 (13). Additional DNA found in pHCM1 but not in R27 was analyzed in detail by using a bioinformatic approach. In pHCM1 there are five regions not present in R27 (Fig. 1). At least 18 of the coding sequences (CDSs) involved are predicted to be involved with resistance to antimicrobial agents or heavy metals, and 16 CDSs (35%) are of unknown function.
FIG.1.
Regions of pHCM1 which are not present in plasmid R27. (A) Large insertion region (bp 149592 to 184546). (B) Small insertion region (bp 113600 to 128350). (C) pHCM.1 bp 7963 to 10458. This region includes five CDSs, HCM1.25 to HCM1.29. (D) pHCM1 bp 77107 to 81205. This region includes three CDSs, HCM1.102 to HCM1.104. (E) pHCM1 bp 141607 to 142983. This region includes CDSs HCMI.192 and HCM1.193. The lines on the outer ring indicate cut sites for HindIII. The insert of region A begins at bp 143085 and ends at bp 184438 on the Salmonella serovar Typhi genome of CT18. The insert of region B begins at bp 13598 and ends at bp 128164. IS10-L, IS10 left element.
Resistance genes.
On pHCM1, CDS HCM1.206 is identical to several reported chloramphenicol acetyltransferase genes (cat) (such as GenBank accession no. P00483) and represents a Tn9 element. HCM1.243 is identical to tetR (accession no. P04483), and HCM1.243 is identical to tetA (accession no. P02980). Both CDSs are associated with tetracycline resistance and represent a Tn10 element. HCM1.240 shows similarity with tetC (accession no. P28815) along 102 of 197 amino acids but is truncated at the 5′ end, where it lacks a recognizable start codon, suggesting that this CDS is not able to form a functional protein and represents a pseudogene. Although tetD is present in R27, it is entirely missing from pHCM1. There is, therefore, similarity with Tn10 from the IS10 left element to midway through tetC. CDS HCM1.222 exhibits 99% homology over the full length of its 816 bp to the sulfonamide resistance gene dihydropteroate synthase (sul-2) from the STX element of Vibrio cholerae (accession no. AY055428) and also shows extensive alignment with the Su-Sm region from RSF1010 (accession no. M28829). CDS HCM1.165 has identity with the product of the Salmonella serovar Typhimurium DT104 plasmid-carried trimethoprim resistance gene dfrA14 (accession no. Z50804) over 155 out of 156 amino acids. HCM1.216 is a putative TEM-type β-lactamase and has 100.0% identity over 286 amino acids with an E. coli β-lactamase precursor encoded by several plasmids (accession no. J01749). Two apparently intact copies of a mercury resistance operon were identified. The first mercury operon (CDSs 1.151c, 1.152, 1.153, 1.157, 1.158, and 1.159) shows similarity to the Pseudomonas aeruginosa mercury resistance operon from plasmid pVS1 (80 to 90%) and with Tn1696. The products of the second mercury resistance operon (CDSs 1.230c, 1.231c, 1.232c, 1.233c, 1.234c, and 1.235c) show very high (80 to 100%) identity in their amino acid sequences to those encoded by a mercury resistance operon from the Shigella plasmid R100 (10), and these sequences include the adjacent tniA delta (HCM1.226c) and the tnpA delta.
Molecular context of the resistance genes.
Unlike R27, pHCM1 has two main regions of apparent DNA insertions which contain all of the resistance genes. The larger region contains 34.955 kbp (pHCM1 bp 149592 to 184546) and is located between CDSs R0139 and R0140 in the R27 sequence. This region includes a partial Tn10 element and CDSs HCM1.195 to HCM1.197, which have homologues in R27, although the sequence is inverted in pHCM1. The smaller region contains 14.751 kbp (pHCM1 bp 113600 to 128350) and is located between CDSs R0168 and R0169 in R27. The larger region appears to have evolved by multiple insertion events (13) at one end, and bordering the region of homology with R27 is a partial Tn10 element. In R27 there is a complete Tn10 element present, but this element is inserted at a different site. In pHCM1 the partial Tn10 element retains tetR, tetA, and a truncated tetC but has lost tetD and the IS10 right element. At the other end of this large insertion region is a copy of the IS10 left element. The IS10 left element is known to be inactive, so the two IS10 sequences do not represent two ends of a Tn10 transposon but rather two separate Tn10 insertion events followed by spontaneous deletion. Such deletion events are usually accompanied by the inversion or deletion of adjacent DNA, and this was indeed found to be the case. CDSs RO140 to RO143 from R27, although shared with pHCM1 (HCM1.195 to HCM1.197) and adjacent to the large insertion, have been inverted. Furthermore, CDSs RO144 to RO148 from R27, which include the citrate utilization genes, have been deleted. It therefore seems possible that there have been insertions of two Tn10 elements into this region of a pHCM1-like plasmid followed by deletion or inversion events and further insertions of mobile elements flanked by other insertion elements (IS elements) (13). Also within this larger insertion region there are two putative pHCM1 replication proteins, HCM1.220 and HCM1.221, not encoded by R27. A total of six CDSs which encode proteins showing similarity with known replication initiation proteins were identified on pHCM1. Four of these, HCM1.54, HCM1.64, HCM1.87, and HCM1.137, are identical to replication genes identified in the sequence of R27, suggesting a common mechanism of replication, in agreement with compatibility data. The sequences for the two genes found only in pHCM1 have similarity with the broad-host-range incQ plasmid RSF1010 (accession no. M28829). HCM1.220 has a 100% match over 717 bases to RepA, but the amino terminus is truncated, and this CDS appears to represent a pseudogene. Over the full length of its CDS (851 bp), HCM1.221 shows 99% similarity to the RepC replication protein from the same plasmid. The presence of multiple replication proteins may go some way to explain the observation of multiple incompatibility groups in MDR plasmids from Salmonella serovar Typhi (11).
The borders of the smaller site of insertion relative to that in R27 show no repeat sequences suggestive of transposition. There is, however, an In4-like integron structure flanked by inverse repeats within Tn1696 (14). There is also a dfrA14 trimethoprim resistance cassette, and although the region immediately to the left of dfrA14 (HCM1.166 to HCM1.168) represents a 5′ conserved sequence typical of class 1 integrons (16), there is no 3′ conserved sequence. With the exception of dfrA14, there is almost 100% identity over this region (pHCM1 bp 117230 to 125910) with regions of plasmid R1033 (accession no. U12338). Although integration sites for the dfrA14 gene cassette are not well defined, these data suggest that acquisition of a class 1 integron was the source for the trimethoprim resistance gene (15). The mercury resistance operon in this region is also part of R1033.
Antimicrobial resistance and plasmid profiles of Salmonella serovar Typhi strains isolated in the same geographical region as Salmonella serovar Typhi CT18(pHCM1).
Salmonella serovar Typhi CT18(pHCM1) was originally isolated at the laboratories of the CTD, Ho Chi Minh City, Vietnam, in December 1993. At the CTD the frequencies of isolation of MDR Salmonella serovar Typhi strains (resistant to chloramphenicol, ampicillin, trimethoprim, sulfamethoxazole, and tetracycline) from blood cultures from patients from southern Vietnam for the indicated year(s) were as follows: 2 of 100 (2%) (1989 to 1991); 35 of 128 (27%) (1992); 117 of 195 (60%) (1993); 311 of 414 (75%) (1994); 618 of 692 (89%) (1995); and 243 of 311 (78%) (1996).
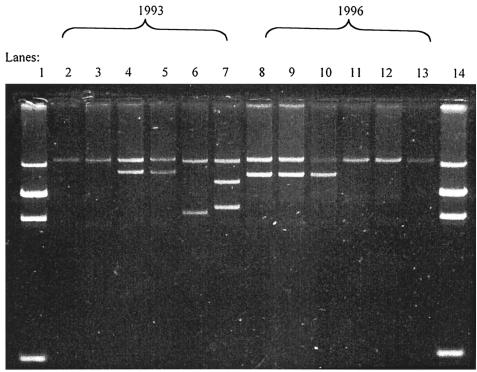
Selected MDR and sensitive Salmonella serovar Typhi strains isolated at the CTD during this period were analyzed for plasmid content and the ability to transfer antibiotic resistance. All MDR Salmonella serovar Typhi isolates (24 isolates from sporadic cases during 1993, 24 from 1996, and 12 from outbreaks) harbored high-molecular-weight plasmids with mobilities in agarose gels close to that of pHCM1 (218 kbp). There were also smaller, cryptic plasmids present within the Salmonella serovar Typhi population (Fig. 2). Transfer of the full MDR phenotype from Salmonella serovar Typhi to E. coli K-12 was achieved in 17 of 24 (71%) MDR isolates from 1993 and 23 of 24 (96%) MDR isolates from 1996 (Table 2). These successful transfers were always associated with the transfer of a plasmid of around 218 kbp. The MICs of antibiotics and mercury for the donor Salmonella serovar Typhi strain were always within 1 dilution of those of their transconjugates.
FIG. 2.
Plasmids from representative MDR Salmonella serovar Typhi strains isolated in 1993 and 1996. Lane 1 contains E. coli 39R size markers of 98, 24, 24, and 4.5 MDa. (This gives a predicted mass of around 140 MDa; however, the gel indicates that the plasmids have masses of around 110 MDa.) Lanes 2 to 7 contain MDR Salmonella serovar Typhi from 1993. Lanes 8 to 13 contain MDR Salmonella serovar Typhi from 1996. Lane 14 contains E. coli 39R.
TABLE 2.
Plasmid profiles and restriction patterns of the plasmids from MDR Salmonella serovar Typhi strains isolated from patients with typhoid fever (sporadic cases and outbreaks)
| Isolation date | Size(s) of plasmid(s) (MDa) | RFLP patterna | Sporadic case (S) or outbreak (O) | Location of patient with typhoid fever (no. of occurrences) | Total no. of occurrences |
|---|---|---|---|---|---|
| 1992-1994 | 110 | 1 | S | Long An (1), Tien Giang (1) | 2 |
| 1992-1994 | 110, 80 | 1 | S | An Giang (1), Dong Thap (1), Tien Giang (3), Vinh Long (1) | 6 |
| 1992-1994 | 110, 85 | 1 | S | Ho Chi Minh City district 5 (1) | 1 |
| 1992-1994 | 110 | 2 | S | Ho Chi Minh City district 6 (2) | 2 |
| 1992-1994 | 110 | 3 | S | Long An (1) | 1 |
| 1992-1994 | 110, 60, 40, 4 | 4 | S | Dong Thap (1) | 1 |
| 1992-1994 | 110, 80 | 5 | S | An Giang (1) | 1 |
| 1992-1994 | 110 | 6 | S | Ho Chi Minh City district 4 (1), Tien Giang (1) | 2 |
| 1992-1994 | 110 | 7 | S | Ho Chi Minh City district 6 (1) | 1 |
| 1996 | 110 | 7 | S | Dong Nai (1), Dong Thap (1), Tien Giang (1), Vinh Long district (1), Ho Chi Minh City districts 1 (1), 4 (1), and 8 (3) | 9 |
| 1996 | 110, 40 | 7 | S | Tien Giang (1), Liong An (2) | 3 |
| 1996 | 110, 80 | 7 | S | Tien Gang (1), Ho Chi Minh City districts 5 (1), 6 (2), and 8 (3) | 7 |
| 1996 | 110, 85 | 7 | S | Cai Be (1), Ho Chi Minh City district 4 (2), Noi Be (1) | 4 |
| 1996 | 110 | NT | S | Ho Chi Minh City district 8 (1) | 1 |
| 1992-1994 | 110 | NT | S | Tien Giang (1) | 1 |
| 1992-1994 | 110, 80 | NT | S | Ho Chi Minh City district 6 (1) | 1 |
| 1992-1994 | 110, 85 | NT | S | Thuan Hai (1) | 1 |
| 1992-1994 | 110, 60, 40, 3 | NT | S | Dong Thap (1) | 1 |
| 1992-1994 | 110, 70, 30, 10, 3 | NT | S | Long An (1) | 1 |
| 1992-1994 | 110, 70, 40, 3 | NT | S | Ho Chi Minh City district 10 (1), Binh Chanh (1) | 2 |
| 1993 | 110, 80 | 1 | O | Kien Giang (3) | 3 |
| 1994 | 110 | 7 | O | Cai Be (2), Thu Thiem (3) | 5 |
| 1996-1997 | 110 | 7 | O | Ho Chi Minh City district 6 (1) | 1 |
| 1994 | 110, 80 | 7 | O | Cai Be (1) | 1 |
| 1996-1997 | 110, 80 | 7 | O | Ho Chi Minh City district 6 (2) | 2 |
Restriction endonuclease analysis of transconjugant plasmids from MDR Salmonella serovar Typhi.
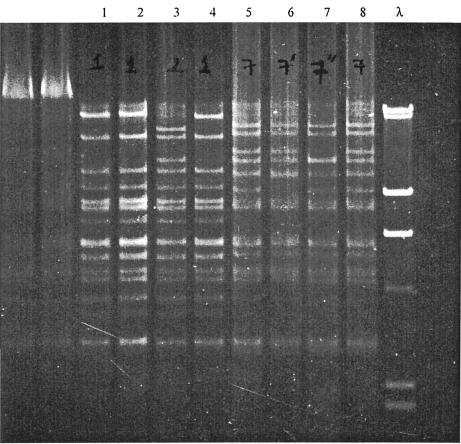
Restriction endonuclease analysis was performed on purified DNA derived from E. coli transconjugates that displayed the full MDR phenotype. The MDR plasmids from 1993 to 1994 generated at least seven RFLPs, which were arbitrarily assigned RFLP numbers 1 to 7 based on how related the patterns appeared on visual inspection of the gels (Fig. 3, Table 2 under “RFLP pattern,” and Table 3). Plasmid pHCM1 generated a pattern designated RFLP1, which was the most common restriction pattern (10 of 17 strains) in this group. Unlike the isolates from 1993 to 1994, all plasmids analyzed from Salmonella serovar Typhi isolated in 1996 generated a conserved restriction profile, which varied by only one detectable DNA fragment from the RFLP7 from 1994. Thus, these patterns were all designated RFLP7 (Fig. 3 and Table 2 under “RFLP pattern”).
FIG. 3.
Restriction patterns of MDR plasmids cleaved with HindIII to show differences between RFLP1, the most common pre-1994 pattern, and RFLP7, the predominant post-1994 pattern. Lanes 1, 2, and 4, pattern 1 Salmonella serovar Typhi strains isolated in 1993; lane 3, pattern 2 Salmonella serovar Typhi strain isolated in 1993; lanes 5 and 8, pattern 7 Salmonella serovar Typhi strains isolated in 1996; lane 6, pattern 7′ plasmid isolated in 1996; lane 7, pattern 7′′ plasmid isolated in 1996; lane λ, DNA markers at 23, 9.4, 6.5, 4.3, 2.3, and 2.0 kb.
TABLE 3.
Diagrammatic representation of the presence of restriction pattern of MDR plasmid from Salmonella serovar Typhi
| DNA fragment | Presence of fragment on original gel at kba:
|
Total | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.0 | 2.3 | 2.9 | 3.6 | 3.9 | 4.3 | 4.4 | 4.7 | 5.3 | 5.8 | 6.1 | 6.5 | 7.2 | 7.2 | 8.0 | 8.4 | 9.4 | 9.5 | 9.8 | 10 | 11 | 11 | 13 | 14 | 17 | 18 | 19 | 19 | 20 | 22 | 23 | 29 | ||
| Control | * | * | * | * | * | * | |||||||||||||||||||||||||||
| 1 | * | * | * | * | * | * | * | * | * | * | * | * | * | * | 114 | ||||||||||||||||||
| 2 | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | 124 | |||||||||||||||||
| 3 | * | * | * | * | * | * | * | * | * | * | * | * | * | * | 108 | ||||||||||||||||||
| 4 | * | * | * | * | * | * | * | * | * | * | * | * | * | * | 126 | ||||||||||||||||||
| 5 | * | * | * | * | * | * | * | * | * | * | * | * | * | * | 111 | ||||||||||||||||||
| 6 | * | * | * | * | * | * | * | * | * | * | * | * | * | * | 112 | ||||||||||||||||||
| 7 | * | * | * | * | * | * | * | * | * | * | * | * | * | * | 128 | ||||||||||||||||||
| 7′ | * | * | * | * | * | * | * | * | * | * | * | * | * | 118 | |||||||||||||||||||
| 7" | * | * | * | * | * | * | * | * | * | * | * | * | * | 114 | |||||||||||||||||||
The position of the DNA fragment are indicated by asterisks.
To investigate the distribution of plasmids with different RFLP patterns, Salmonella serovar Typhi isolates from four well-characterized typhoid outbreaks (5) were analyzed. Plasmids from MDR Salmonella serovar Typhi associated with outbreaks in 1993 were RFLP1, whereas similar isolates from different outbreaks in 1994 and 1996 were RFLP7 (Table 2). There were differences between the RFLP7 plasmids and the earlier RFLP1 plasmids isolated before 1994 in at least 50% of the distinguishable restriction fragments (Fig. 3 and Table 3).
Multiplex PCR for resistance genes.
To identify any variation in resistance genes encoding the MDR phenotype in clinical isolates of Salmonella serovar Typhi, a multiplex PCR was developed. All MDR Salmonella serovar Typhi isolates from 1993 harbored the same resistance genes as pHCM1 as well as an extra sulfonamide resistance gene, sul-1. The plasmid pHCM1 DNA template generated positive PCR products with the sul-2-specific primers but not with the sul-1-specific primers. MDR plasmids exhibiting the RFLP7 pattern harbored the same two sulfonamide resistance genes as well as a different trimethoprim resistance gene, dfrA7, encoding dihydrofolate reductase VII. Furthermore, DNA from two RFLP7 plasmids isolated in 1996 was found by PCR analysis to carry tetRACD (data not shown). In earlier plasmids isolated before 1994, there was a truncation midway through tetC. These data confirm that the plasmids, although of similar sizes and despite conferring the same resistance phenotype, were genetically distinct, a finding consistent with the restriction enzyme analysis. At least one product of the antimicrobial resistance genes from each PCR was sequenced. All gave results consistent with amplification of the specific target gene.
DISCUSSION
Multiple antimicrobial resistance in Salmonella serovar Typhi in southern Vietnam was first reported, anecdotally, in the late 1980s. In 1992 MDR was still rare, found in only 2% of 100 Salmonella serovar Typhi isolates from the southern provinces, but by 1995 it was very common. Almost 90% of blood culture isolates were MDR. Although the resistance plasmids identified from resistant Salmonella serovar Typhi in Vietnam have all been of the incH group and greater than 190 kbp (2, 18, 20, 24), we have detected genetic variation in the MDR plasmid population and have identified the emergence of a distinct MDR plasmid genotype, RFLP7. Comparison of the DNA sequence of pHCM1, isolated in 1993 from Salmonella serovar Typhi in Vietnam, with the prototype incH plasmid, R27, isolated in 1961 from S. enterica in the United Kingdom, revealed extensive regions of homology and two regions of apparent insertion in pHCM1 that R27 does not have. These regions are hot spots for variation. There are 46 predicted CDSs in these two regions of acquired genes in pHCM1 that are not present in R27, and of these acquired genes, 18 were predicted to encode resistance to antimicrobial agents or heavy metals. These genes may provide a selective advantage for plasmid carriage by Salmonella serovar Typhi within the human host in the presence of antimicrobial agents or in the presence of mercury in the human host or environment (19). Interestingly, a DNA arrangement similar to the larger insertion on pHCM11 is present on plasmid R100 (NR1), which has a transposon (Tn21) encoding mercury resistance inserted into a Tn9-like element encoding chloramphenicol resistance (10). In pHCM1 several pairs of IS elements are present, suggesting that acquisition has occurred by insertion within an insertion of transposon-like elements. At the borders of this region are two identical transposase-inactive IS10 left elements. One explanation for the presence of these elements is acquisition of DNA by Tn10 transposition followed by the loss of the active transposase of the IS10 right element. The presence of the Tn10 elements may have facilitated the occurrence of other insertion events involving more IS elements, thus defining this region of the plasmid as a site of DNA acquisition.
The smaller of the two insertion regions on pHCM1 harbors a single antimicrobial resistance gene, dfrA14, encoding trimethoprim resistance embedded in a 7.5-kb sequence which resembles a region on plasmid R1033. There is a complete integron integrase (intI1) present in this region, adjacent to dfr, and we have demonstrated the presence of class 1 integrase (intI1) in all of the MDR plasmids tested in this study as well as the presence of sul-1 in all plasmids except pHCM1. The exact location of sul-1 is unknown, but its presence suggests that class 1 integrons may be present in most of these resistance plasmids and that some may have 3′ conserved regions. Class 1 integron acquisition probably represents an important mode of acquisition of trimethoprim resistance in Salmonella serovar Typhi, but the exact nature of the gene cassettes involved remains to be elucidated from sequence analysis of earlier plasmids in this series. The gene(s) encoding trimethoprim resistance was the last to be acquired by MDR Salmonella serovar Typhi. Therefore, the presence of the dfr gene in a region within plasmid pHCM1 different from that of other resistance genes goes some way toward explaining the historical data.
In this study, MDR plasmids isolated before 1994 gave several different restriction patterns (RFLP1 to RFLP7), whereas after 1994 plasmids with a single RFLP pattern, RFLP7, were predominant. The variations in the RFLP patterns were mapped, by using sequence data, to the two regions of insertion described above. We now know that some of the differences in RFLP patterns are caused by the acquisition of different resistance genes and by insertion events involving transposon- and integron-like structures. The apparent reduction in genetic variation in the MDR plasmids isolated after 1994 compared with that of those isolated before 1994 may be due to a single successful Salmonella serovar Typhi host spreading a single plasmid type, or alternatively, a single successful plasmid type may be propagating in several hosts. If the latter is true, then enhanced transmission of disease may well be a property conferred on Salmonella serovar Typhi by the MDR plasmid (23).
Our data from this and previous work suggest that the MDR phenotype of Salmonella serovar Typhi in a region where typhoid is endemic is caused by the spread of R27-like plasmids in several different bacterial strains. It has previously been shown that there is variation in the chromosome of Salmonella serovar Typhi causing MDR typhoid fever in this region (5). In this study, we have shown that several of the same isolates previously shown by whole-chromosome pulsed-field gel electrophoresis to vary harbor plasmids with a single RFLP pattern. It seems likely, therefore, that across southern Vietnam a single successful plasmid is spreading into different Salmonella serovar Typhi genotypes. The R27-like plasmids have evolved by the serial acquisition of DNA on mobile elements encoding resistance to antimicrobials and heavy metals and carrying several genes of unknown function. Before 1994, these plasmids showed variation in their RFLP patterns, which was caused by differences in the genetic contents of two variable regions. Since 1994, there has been a predominant plasmid RFLP type found in different Salmonella serovar Typhi strains. This apparently enhanced stability of plasmid structure seems to be a property of the plasmid rather than the bacterial host. A fuller understanding of this process of plasmid-host adaptation may be achieved by further studying these plasmids, which may shed light on the mechanism for the maintenance of plasmid-borne antibiotic resistance genes in bacterial populations.
Acknowledgments
We acknowledge the support of the technical and medical staff of the Microbiology Laboratories and the clinical staff and the directors of the Centre for Tropical Diseases and Dong Thap Provincial Hospital, Dong Thap, Vietnam. Thanks are due also to J. Threlfall for advice and to Trevor Lawley for providing pR27 DNA.
This work was supported by The Wellcome Trust of the United Kingdom.
REFERENCES
- 1.Acar, J., E. Kaplan, and T. O'Brian. 1997. Monitoring and management of bacterial resistance to antimicrobial agents: a World Health Organization symposium. Clin. Infect. Dis. 24(Suppl. 1):S1-S176. [PubMed] [Google Scholar]
- 2.Anonymous. 1982. The geographical distribution of Salmonella typhi and Salmonella paratyphi A and B phage types during the period 1 January 1970 to 31 December 1973. A report of the International Federation for Enteric Phage-Typing (IFEPT). J. Hyg. 88:231-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausable, F. M., R. Brent, and R. E. Kingston (ed.). 1996. Current protocols in molecular biology. John Wiley and Sons, New York, N.Y.
- 4.Cohen, L. C. 1992. Epidemiology of drug resistance: implications for a post-antimicrobial era. Science 257:1050-1055. [DOI] [PubMed] [Google Scholar]
- 5.Connerton, P., J. Wain, T. T. Hien, T. Ali, C. Parry, N. T. Chinh, H. Vinh, V. A. Ho, T. S. Diep, N. P. J. Day, N. J. White, G. Dougan, and J. J. Farrar. 2000. Epidemic typhoid in Vietnam: molecular typing of multiple-antibiotic-resistant Salmonella enterica serotype Typhi from four outbreaks. J. Clin. Microbiol. 38:895-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillespie, S. H. 2001. Antibiotic resistance in the absence of selective pressure. Int. J. Antimicrob. Agents 17:171-176. [DOI] [PubMed] [Google Scholar]
- 7.Gomez-Lus, R. 1998. Evolution of bacterial resistance to antibiotics during the last three decades. Int. Microbiol. 1:279-284. [PubMed] [Google Scholar]
- 8.Hampton, M. D., L. R. Ward, B. Rowe, and E. J. Threlfall. 1998. Molecular fingerprinting of multidrug-resistant Salmonella enterica serotype Typhi. Emerg. Infect. Dis. 4:317-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kado, C. I., and S.-T. Liu. 1981. Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol. 145:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liebert, C. A., R. M. Hall, and A. O. Summers. 1999. Transposon Tn21, flagship of the floating genome. Microbiol. Mol. Biol. Rev. 63:507-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mirza, S., S. Kariuki, K. Z. Mamun, N. J. Beeching, and C. A. Hart. 2000. Analysis of plasmid and chromosomal DNA of multidrug-resistant Salmonella enterica serovar Typhi from Asia. J. Clin. Microbiol. 38:1449-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NCCLS. 1999. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M07-A5. (Updated 2000.) NCCLS, Wayne, Pa.
- 13.Parkhill, J., G. Dougan, K. D. James, N. R. Thomson, D. Pickard, J. Wain, C. Churcher, K. L. Mungall, S. D. Bentley, M. T. Holden, M. Sebaihia, S. Baker, D. Basham, K. Brooks, T. Chillingworth, P. Connerton, A. Cronin, P. Davis, R. M. Davies, L. Dowd, N. White, J. Farrar, T. Feltwell, N. Hamlin, A. Haque, T. T. Hien, S. Holroyd, K. Jagels, A. Krogh, T. S. Larsen, S. Leather, S. Moule, P. O'Gaora, C. Parry, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 413:848-852. [DOI] [PubMed] [Google Scholar]
- 14.Partridge, S. R., G. D. Recchia, H. W. Stokes, and R. M. Hall. 2001. Family of class 1 integrons related to In4 from Tn1696. Antimicrob. Agents Chemother. 45:3014-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ploy, M.-C., D. Chainier, N. H. Tran Thi, I. Poilane, P. Cruaud, F. Denis, A. Collignon, and T. Lambert. 2003. Integron-associated antibiotic resistance in Salmonella enterica serovar Typhi from Asia. Antimicrob. Agents Chemother. 47:1427-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Recchia, G. D., and R. M. Hall. 1995. Gene cassettes: a new class of mobile element. Microbiology (Reading) 141:3015-3027. [DOI] [PubMed] [Google Scholar]
- 17.Sherburne, C. K., T. D. Lawley, M. W. Gilmour, F. R. Blattner, V. Burland, E. Grotbeck, D. J. Rose, and D. E. Taylor. 2000. The complete DNA sequence and analysis of R27, a large IncHI plasmid from Salmonella typhi that is temperature sensitive for transfer. Nucleic Acids Res. 28:2177-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith, H. W., Z. Parsell, and P. Green. 1978. Thermosensitive H1 plasmids determining citrate utilization. J. Gen. Microbiol. 109:305-311. [DOI] [PubMed] [Google Scholar]
- 19.Summers, A. O., J. Wireman, M. J. Vimy, F. L. Lorscheider, B. Marshall, S. B. Levy, S. Bennett, and L. Billard. 1993. Mercury released from dental “silver” fillings provokes an increase in mercury- and antibiotic-resistant bacteria in oral and intestinal floras of primates. Antimicrob. Agents Chemother. 37:825-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor, D. E., and E. C. Brose. 1985. Characterization of incompatibility group HI1 plasmids from Salmonella typhi by restriction endonuclease digestion and hybridization of DNA probes for Tn3, Tn9, and Tn10. Can. J. Microbiol. 31:721-729. [DOI] [PubMed] [Google Scholar]
- 21.Tenover, F. C. 1995. The best of times, the worst of times. The global challenge of antimicrobial resistance. Pharm. World Sci. 17:149-151. [DOI] [PubMed] [Google Scholar]
- 22.Tran, T. H., D. B. Bethell, T. T. Nguyen, J. Wain, S. D. To, T. P. Le, M. C. Bui, M. D. Nguyen, T. T. Pham, A. L. Walsh, et al. 1995. Short course of ofloxacin for treatment of multidrug-resistant typhoid. Clin. Infect. Dis. 20:917-923. [PubMed] [Google Scholar]
- 23.Wain, J., T. S. Diep, V. A. Ho, A. M. Walsh, T. T. H. Nguyen, C. M. Parry, and N. J. White. 1998. Quantitation of bacteria in blood of typhoid fever patients and relationship between counts and clinical features, transmissibility, and antibiotic resistance. J. Clin. Microbiol. 36:1683-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wain, J., T. T. Hien, P. Connerton, T. Ali, C. M Parry, N. T. T. Chinh, H. Vinh, C. X. T. Phuong, V. A. Ho, T. S. Diep, J. J. Farrar, N. J. White, and G. Dougan. 1999. Molecular typing of multiple-antibiotic-resistant Salmonella enterica serovar Typhi from Vietnam: application to acute and relapse cases of typhoid fever. J. Clin. Microbiol. 37:2466-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White, N. 1999. Salmonella typhi and paratyphi. In L. Yu, C. Merigan, and S. Barriere (ed.), Antimicrobial therapy and vaccines. Williams and Wilkins, Baltimore, Md.