Abstract
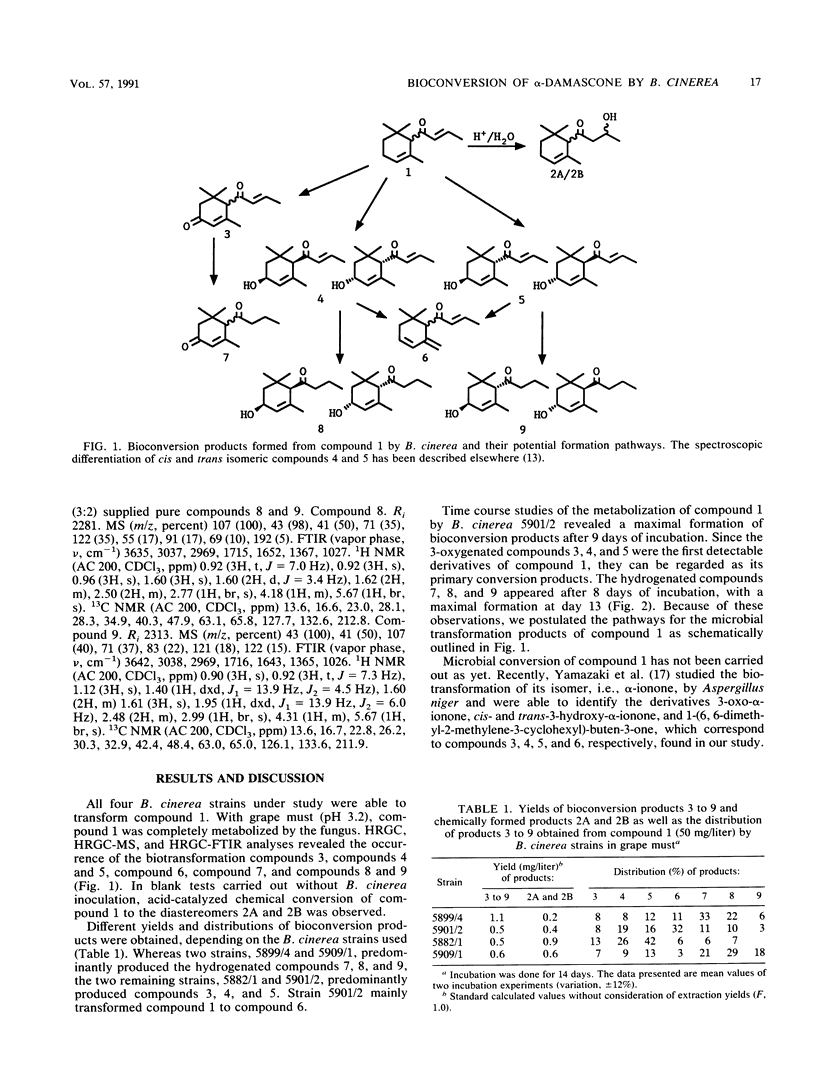
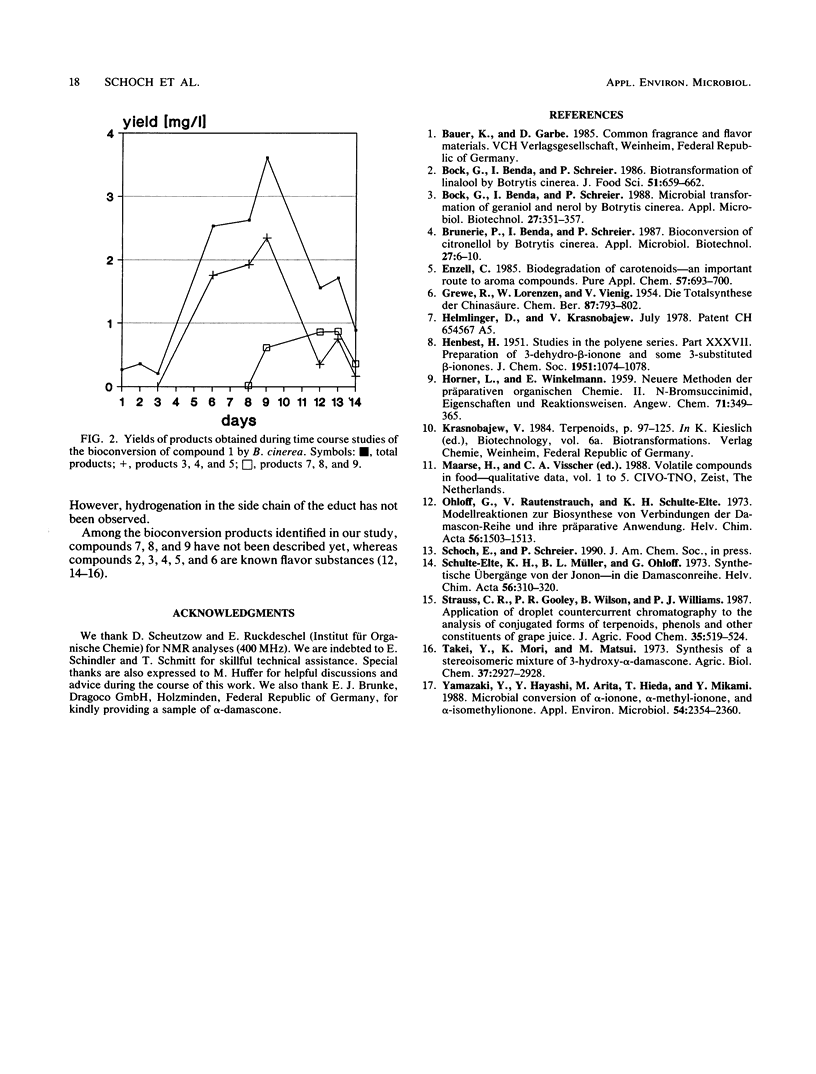
Bioconversion of α-damascone (compound 1) was studied with four strains of Botrytis cinerea in grape must (pH 3.2). As biotransformation products of compound 1, 3-oxo-α-damascone, cis- and trans-3-hydroxy-α-damascone, γ-damascenone, 3-oxo-8, 9-dihydro-α-damascone, and cis- and trans-3-hydroxy-8,9-dihydro-α-damascone were identified. In addition, acid-catalyzed chemical transformation of compound 1 to the diastereomers of 9-hydroxy-8,9-dihydro-α-damascone was observed. Identifications were performed by capillary gas chromatography (HRGC) and coupled HRGC techniques, i.e., on-line HRGC-mass spectrometry and HRGC-Fourier transform infrared spectroscopy, after extractive sample preparation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Yamazaki Y., Hayashi Y., Arita M., Hieda T., Mikami Y. Microbial Conversion of alpha-Ionone, alpha-Methylionone, and alpha-Isomethylionone. Appl Environ Microbiol. 1988 Oct;54(10):2354–2360. doi: 10.1128/aem.54.10.2354-2360.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


