Abstract
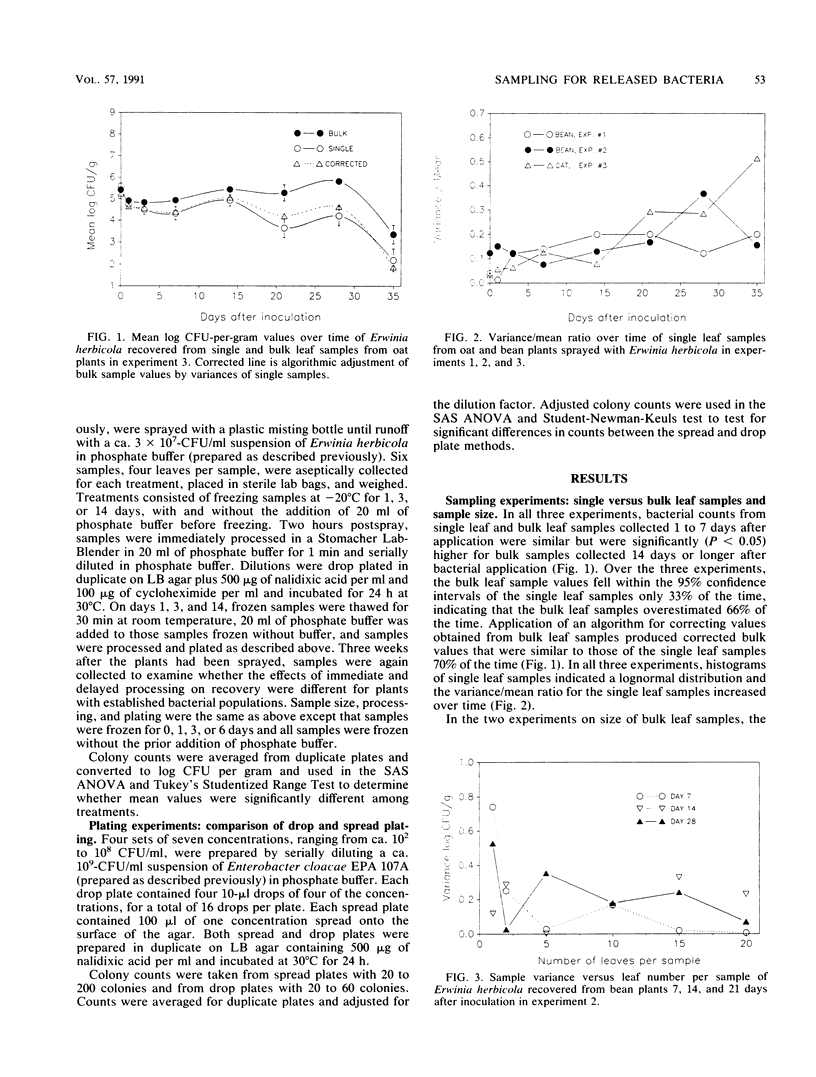
Determining the fate and survival of genetically engineered microorganisms released into the environment requires the development and application of accurate and practical methods of detection and enumeration. Several experiments were performed to examine quantitative recovery methods that are commonly used or that have potential applications. In these experiments, Erwinia herbicola and Enterobacter cloacae were applied in greenhouses to Blue Lake bush beans (Phaseolus vulgaris) and Cayuse oats (Avena sativa). Sampling indicated that the variance in bacterial counts among leaves increased over time and that this increase caused an overestimation of the mean population size by bulk leaf samples relative to single leaf samples. An increase in the number of leaves in a bulk sample, above a minimum number, did not significantly reduce the variance between samples. Experiments evaluating recovery methods demonstrated that recovery of bacteria from leaves was significantly better with stomacher blending, than with blending, sonication, or washing and that the recovery efficiency was constant over a range of sample inoculum densities. Delayed processing of leaf samples, by storage in a freezer, did not significantly lower survival and recovery of microorganisms when storage was short term and leaves were not stored in buffer. The drop plate technique for enumeration of bacteria did not significantly differ from the spread plate method. Results of these sampling, recovery, and enumeration experiments indicate a need for increased development and standardization of methods used by researchers as there are significant differences among, and also important limitations to, some of the methods used.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hirano S. S., Nordheim E. V., Arny D. C., Upper C. D. Lognormal distribution of epiphytic bacterial populations on leaf surfaces. Appl Environ Microbiol. 1982 Sep;44(3):695–700. doi: 10.1128/aem.44.3.695-700.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S. S., Upper C. D. Diel Variation in Population Size and Ice Nucleation Activity of Pseudomonas syringae on Snap Bean Leaflets. Appl Environ Microbiol. 1989 Mar;55(3):623–630. doi: 10.1128/aem.55.3.623-630.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoben H. J., Somasegaran P. Comparison of the Pour, Spread, and Drop Plate Methods for Enumeration of Rhizobium spp. in Inoculants Made from Presterilized Peat. Appl Environ Microbiol. 1982 Nov;44(5):1246–1247. doi: 10.1128/aem.44.5.1246-1247.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen G. R., Walter M. V., Porteous L. A., Prince V. J., Armstrong J. L., Seidler R. J. Predictive model of conjugative plasmid transfer in the rhizosphere and phyllosphere. Appl Environ Microbiol. 1988 Feb;54(2):343–347. doi: 10.1128/aem.54.2.343-347.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann J., Constantinidou H. A., Barchet W. R., Upper C. D. Plants as sources of airborne bacteria, including ice nucleation-active bacteria. Appl Environ Microbiol. 1982 Nov;44(5):1059–1063. doi: 10.1128/aem.44.5.1059-1063.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindow S. E., Arny D. C., Upper C. D. Distribution of ice nucleation-active bacteria on plants in nature. Appl Environ Microbiol. 1978 Dec;36(6):831–838. doi: 10.1128/aem.36.6.831-838.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindow S. E., Knudsen G. R., Seidler R. J., Walter M. V., Lambou V. W., Amy P. S., Schmedding D., Prince V., Hern S. Aerial Dispersal and Epiphytic Survival of Pseudomonas syringae during a Pretest for the Release of Genetically Engineered Strains into the Environment. Appl Environ Microbiol. 1988 Jun;54(6):1557–1563. doi: 10.1128/aem.54.6.1557-1563.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues U. M., Kroll R. G. Microcolony epifluorescence microscopy for selective enumeration of injured bacteria in frozen and heat-treated foods. Appl Environ Microbiol. 1989 Apr;55(4):778–787. doi: 10.1128/aem.55.4.778-787.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagory D., Lindow S. E., Parmeter J. R. Toxicity of smoke to epiphytic ice nucleation-active bacteria. Appl Environ Microbiol. 1983 Jul;46(1):114–119. doi: 10.1128/aem.46.1.114-119.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



