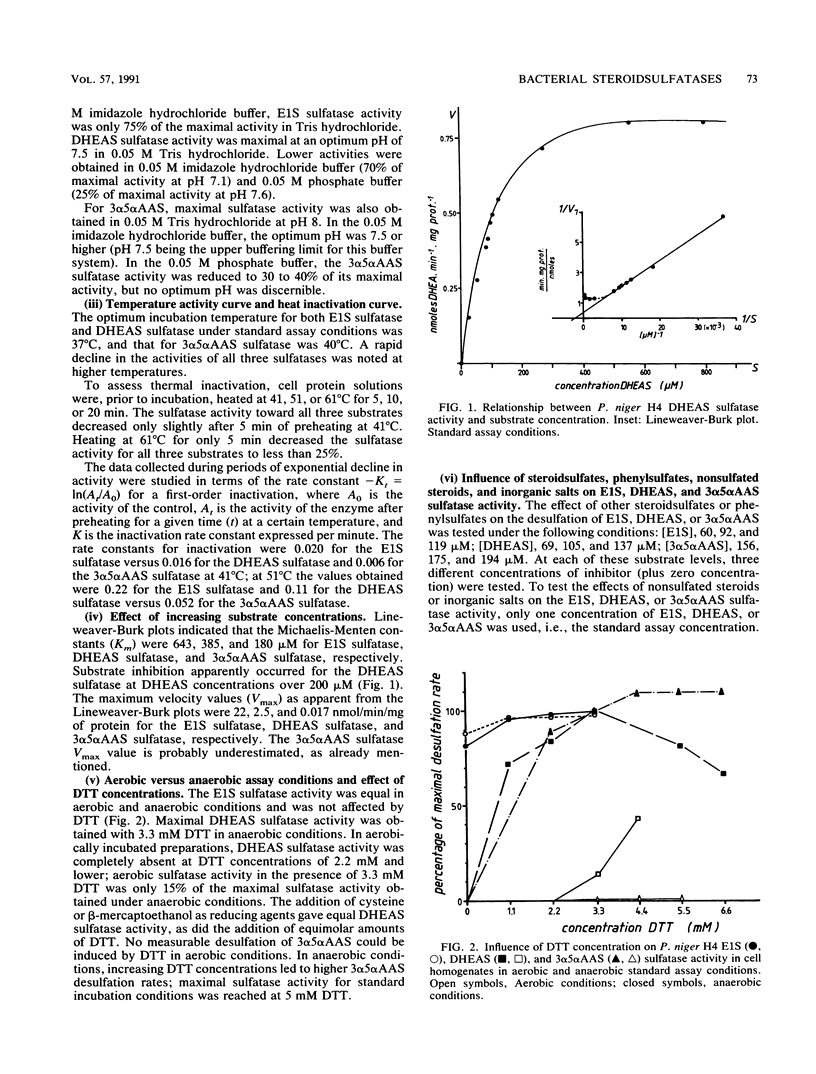
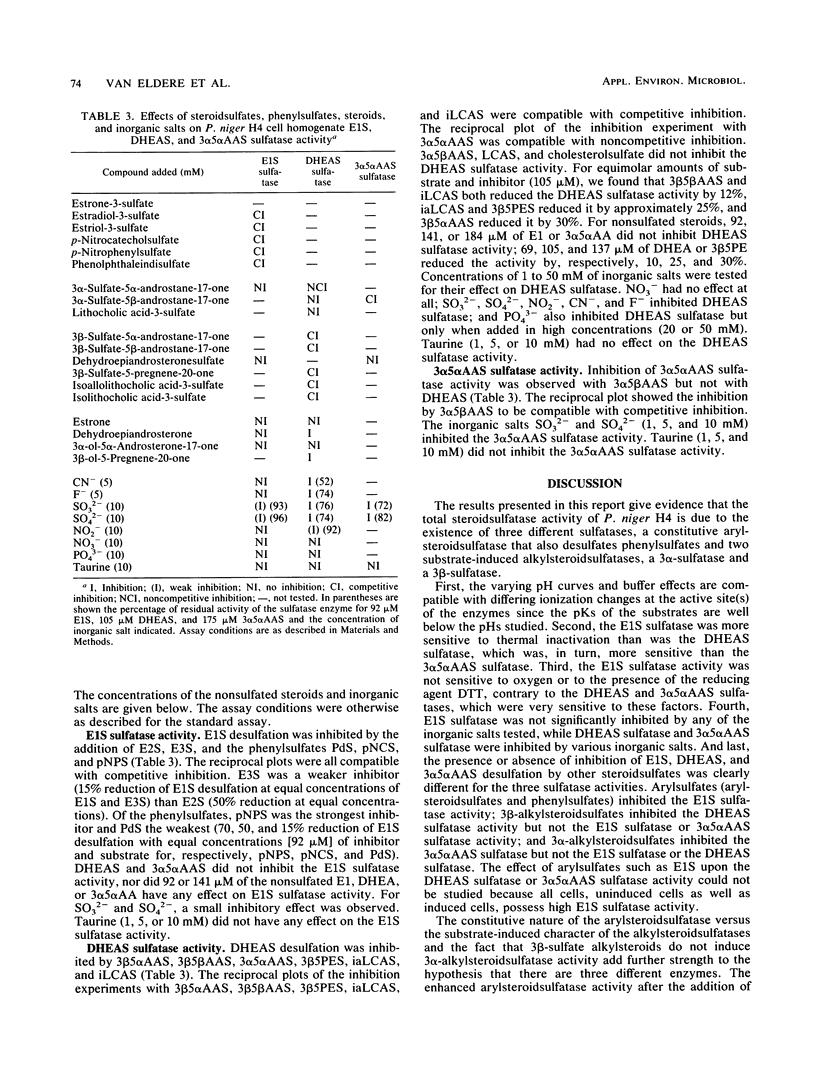
Abstract
The strictly anaerobic intestinal Peptococcus niger H4 synthesizes three different steroidsulfatase enzymes: a constitutive arylsulfatase and two inducible alkylsteroidsulfatases. The arylsulfatase desulfates estrogen-3-sulfates and phenylsulfates. The two alkylsteroidsulfatases desulfate, respectively, 3 alpha-sulfates and 3 beta-sulfates of delta 5, 5 alpha, and 5 beta androstanes, pregnanes, and bile acids. Cholesterol-3 beta-sulfate was not desulfated by the alkylsteroidsulfatases nor were steroids or bile acids that were sulfated in positions other than the 3 position. The alkylsteroidsulfatases were induced by their substrates; bile acid sulfates, however, were poor inducers of the 3 beta-sulfatase and did not induce the 3 alpha-sulfatase activity. In intact bacterial cells, taurine and sulfite suppressed the induction of the alkylsteroidsulfatases and inhibited the activity of the arylsulfatase and alkylsteroidsulfatases. In cell homogenates, the arylsulfatase and alkylsteroidsulfatases activities were inhibited by sulfite and sulfate but not by taurine. Our results support the hypothesis that the main function of the steroidsulfatases in P. niger H4 is to provide the bacteria with sulfur for dissimilatory purposes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bokkenheuser V. D., Winter J., Kelly W. G. Metabolism of biliary steroids by human fecal flora. Am J Clin Nutr. 1978 Oct;31(10 Suppl):S221–S226. doi: 10.1093/ajcn/31.10.S221. [DOI] [PubMed] [Google Scholar]
- Burns G. R., Wynn C. H. Studies on the Arylsulphatase and phenol sulphotransferase activities of Aspergillus oryzae. Biochem J. 1975 Sep;149(3):697–705. doi: 10.1042/bj1490697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S., POWELL G. M. Studies on sulphatases. 27. The purification and properties of the arylsulphatase of the digestive gland of Helix pomatia. Biochem J. 1959 Dec;73:672–679. doi: 10.1042/bj0730672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel W. L. Arylsulfatase C and the steroid sulfatases. Isozymes Curr Top Biol Med Res. 1985;12:189–228. [PubMed] [Google Scholar]
- Eriksson H. Absorption and enterohepatic circulation of neutral steroids in the rat. Eur J Biochem. 1971 Apr;19(3):416–423. doi: 10.1111/j.1432-1033.1971.tb01331.x. [DOI] [PubMed] [Google Scholar]
- Eriksson H., Gustafsson J. A. Steroids in germfree and conventional rats. Sulpho- and glucuronohydrolase activities of caecal contents from conventional rats. Eur J Biochem. 1970 Mar 1;13(1):198–202. doi: 10.1111/j.1432-1033.1970.tb00919.x. [DOI] [PubMed] [Google Scholar]
- Eyssen H. J., Parmentier G. G., Mertens J. A. Sulfate bile acids in germ-free and conventional mice. Eur J Biochem. 1976 Jul 15;66(3):507–514. doi: 10.1111/j.1432-1033.1976.tb10576.x. [DOI] [PubMed] [Google Scholar]
- Eyssen H., Van Eldere J., Parmentier G., Huijghebaert S., Mertens J. Influence of microbial bile salt desulfation upon the fecal excretion of bile salts in gnotobiotic rats. J Steroid Biochem. 1985 Apr;22(4):547–554. doi: 10.1016/0022-4731(85)90176-1. [DOI] [PubMed] [Google Scholar]
- Fitzgerald J. W., Scott C. L. Utilization of choline-o-sulphate as a sulphur source for growth by a Pseudomonas isolate. Microbios. 1974 Apr;10(38):121–131. [PubMed] [Google Scholar]
- France J. T. Steroid sulphatase deficiency. J Steroid Biochem. 1979 Jul;11(1B):647–651. doi: 10.1016/0022-4731(79)90094-3. [DOI] [PubMed] [Google Scholar]
- Francis M. M., Kowalsky N., Watanabe M. Extraction of a steroid transport system from Pseudomonas testosteroni membranes and incorporation into synthetic liposomes. J Steroid Biochem. 1985 Oct;23(4):523–528. doi: 10.1016/0022-4731(85)90202-x. [DOI] [PubMed] [Google Scholar]
- George J. R., Fitzgerald J. W. Tyramine-mediated enhancement of arylsulphatase purified from Pseudomonas C12B [proceedings]. Biochem Soc Trans. 1979 Oct;7(5):1082–1083. doi: 10.1042/bst0071082. [DOI] [PubMed] [Google Scholar]
- Huijghebaert S. M., Mertens J. A., Eyssen H. J. Isolation of a bile salt sulfatase-producing Clostridium strain from rat intestinal microflora. Appl Environ Microbiol. 1982 Jan;43(1):185–192. doi: 10.1128/aem.43.1.185-192.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato T. J., Wong C. G., Chen L. J., Bolt R. J. Hydrolysis of lithocholate sulfate by Pseudomonas aeruginosa. J Bacteriol. 1977 Apr;130(1):545–547. doi: 10.1128/jb.130.1.545-547.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänne O. A., Laatikainen T. J., Vihko R. K. Effect of reduction of the intestinal microflora on the excretion of neutral steroids in human faeces and urine. Eur J Biochem. 1971 May 11;20(1):120–123. doi: 10.1111/j.1432-1033.1971.tb01369.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Orme M. L., Back D. J. Therapy with oral contraceptive steroids and antibiotics. J Antimicrob Chemother. 1979 Mar;5(2):124–126. doi: 10.1093/jac/5.2.124. [DOI] [PubMed] [Google Scholar]
- Pacini N., Albini E., Ferrari A., Zanchi R., Marca G., Bandiera T. Transformation of sulfated bile acids by human intestinal microflora. Arzneimittelforschung. 1987 Aug;37(8):983–987. [PubMed] [Google Scholar]
- Parmentier G., Eyssen H. Synthesis and characteristics of the specific monosulfates of chenodeoxycholate, deoxycholate and their taurine or glycine conjugates. Steroids. 1977 Nov;30(5):583–590. doi: 10.1016/0039-128x(77)90049-6. [DOI] [PubMed] [Google Scholar]
- ROY A. B. The steroid sulphatase of Patella vulgata. Biochem J. 1956 Jan;62(1):41–50. doi: 10.1042/bj0620041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robben J., Parmentier G., Eyssen H. Isolation of a rat intestinal Clostridium strain producing 5 alpha- and 5 beta-bile salt 3 alpha-sulfatase activity. Appl Environ Microbiol. 1986 Jan;51(1):32–38. doi: 10.1128/aem.51.1.32-38.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim S. M., Huijghebaert S., Back D. J., Eyssen H. J. Gastrointestinal absorption of estrone sulfate in germfree and conventional rats. J Steroid Biochem. 1983 Apr;18(4):499–503. doi: 10.1016/0022-4731(83)90071-7. [DOI] [PubMed] [Google Scholar]
- Tikkanen M. J., Pulkkinen M. O., Adlercreutz H. Effect of ampicillin treatment on the urinary excretion of estriol conjugates in pregnancy. J Steroid Biochem. 1973 Jul;4(4):439–440. doi: 10.1016/0022-4731(73)90015-0. [DOI] [PubMed] [Google Scholar]
- Van Eldere J. R., De Pauw G., Eyssen H. J. Steroid sulfatase activity in a Peptococcus niger strain from the human intestinal microflora. Appl Environ Microbiol. 1987 Jul;53(7):1655–1660. doi: 10.1128/aem.53.7.1655-1660.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eldere J., Robben J., De Pauw G., Merckx R., Eyssen H. Isolation and identification of intestinal steroid-desulfating bacteria from rats and humans. Appl Environ Microbiol. 1988 Aug;54(8):2112–2117. doi: 10.1128/aem.54.8.2112-2117.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S., Stiehl A., Raedsch R., Klöters P., Kommerell B. Colonic absorption of sulfated and nonsulfated bile acids in rat. Digestion. 1986;33(1):1–6. doi: 10.1159/000199268. [DOI] [PubMed] [Google Scholar]
- van Eldere J., Parmentier G., Robben J., Eyssen H. Influence of an estrone-desulfating intestinal flora on the enterohepatic circulation of estrone-sulfate in rats. J Steroid Biochem. 1987 Feb;26(2):235–239. doi: 10.1016/0022-4731(87)90077-x. [DOI] [PubMed] [Google Scholar]


