Abstract
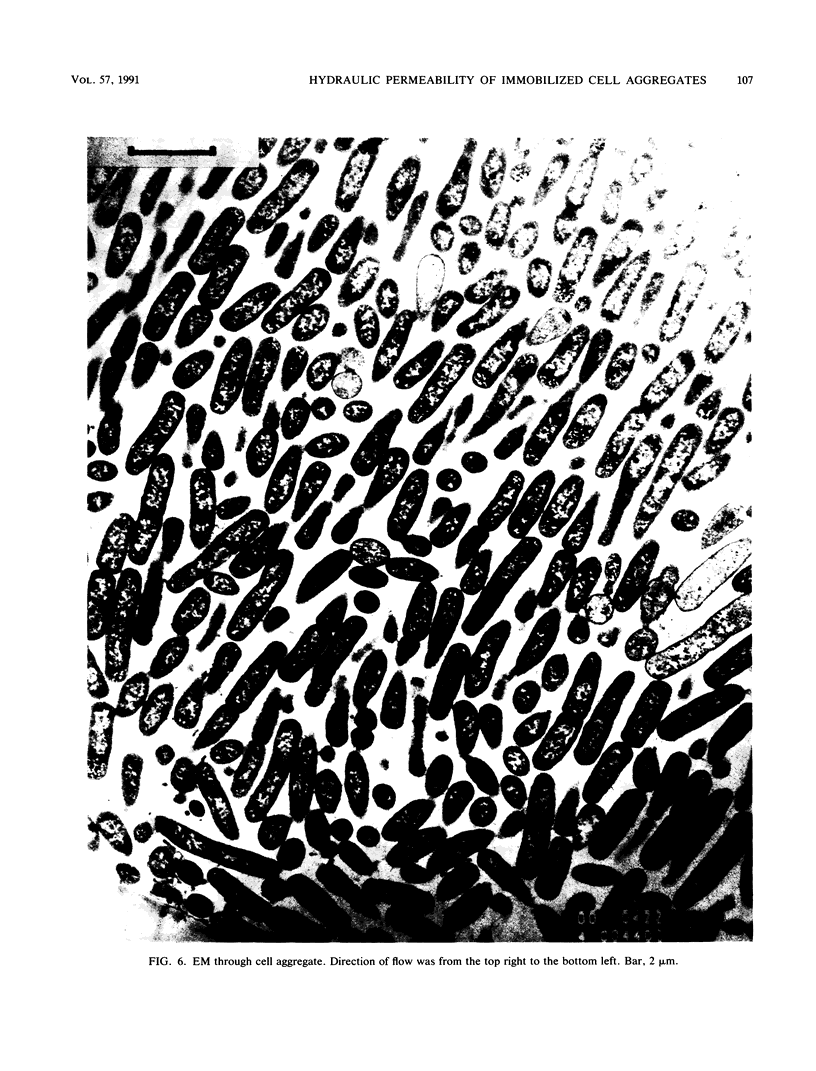
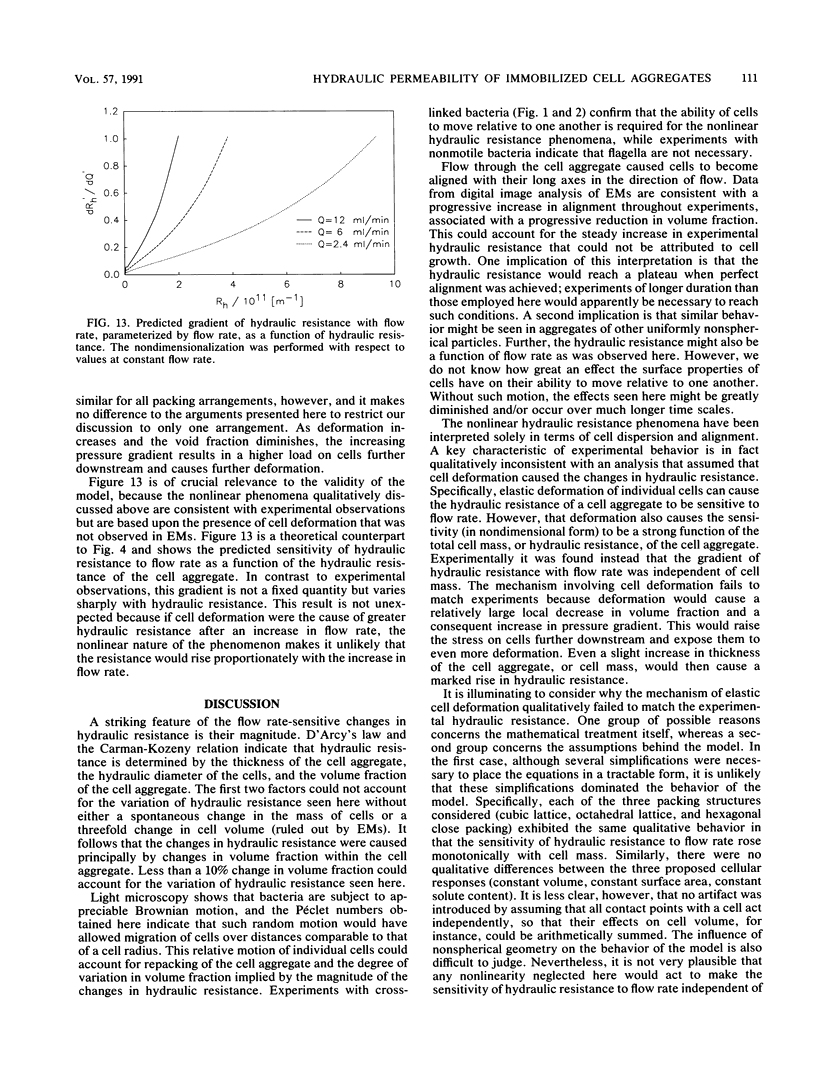
A dense aggregate of cells was retained in a reactor by a supported porous membrane. A continuous flow of nutrient medium was maintained through the cell aggregate and membrane. The hydraulic resistance of the cell aggregate was monitored throughout experiments with either growing or chemically cross-linked cells, under conditions of varying flow rates. Digital image analysis was used to characterize the sizes, separations, and orientations of several thousand individual cells in electron micrographs of chemically cross-linked cell aggregates. Two nonlinear phenomena were observed. First, the hydraulic resistance varied in direct relation to and reversibly with flow rate. Second, in constant flow-rate experiments the hydraulic resistance increased with time at a faster rate than could be attributed to cell growth. Both of these phenomena were dependent upon and could be explained by the ability of cells to move with respect to one another, under the influences of Brownian motion and of convection. Such relative motion could allow changes in net alignment of cells in the direction of flow and in the volume fraction of cells in the aggregate. This explanation is consistent with image analysis data. The observed sensitivity of hydraulic resistance to flow rate was inconsistent with a model that assumed elastic deformation of individual cells, and no evidence of cell deformation was found in electron micrographs.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fowler J. D., Robertson C. R. Metabolic behavior of immobilized aggregates of Escherichia coli under conditions of varying mechanical stress. Appl Environ Microbiol. 1991 Jan;57(1):93–101. doi: 10.1128/aem.57.1.93-101.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L. How bacteria grow and divide in spite of internal hydrostatic pressure. Can J Microbiol. 1985 Dec;31(12):1071–1084. doi: 10.1139/m85-204. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Pinette M. F. Nephelometric determination of turgor pressure in growing gram-negative bacteria. J Bacteriol. 1987 Aug;169(8):3654–3663. doi: 10.1128/jb.169.8.3654-3663.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Rhoads D. B., Epstein W. Osmotic control of kdp operon expression in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Jan;78(1):464–468. doi: 10.1073/pnas.78.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinette M. F., Koch A. L. Turgor pressure responses of a gram-negative bacterium to antibiotic treatment, measured by collapse of gas vesicles. J Bacteriol. 1988 Mar;170(3):1129–1136. doi: 10.1128/jb.170.3.1129-1136.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth W. G., Leckie M. P., Dietzler D. N. Osmotic stress drastically inhibits active transport of carbohydrates by Escherichia coli. Biochem Biophys Res Commun. 1985 Jan 16;126(1):434–441. doi: 10.1016/0006-291x(85)90624-2. [DOI] [PubMed] [Google Scholar]
- Stewart P. S., Robertson C. R. Product inhibition of immobilized Escherichia coli arising from mass transfer limitation. Appl Environ Microbiol. 1988 Oct;54(10):2464–2471. doi: 10.1128/aem.54.10.2464-2471.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]




