Abstract
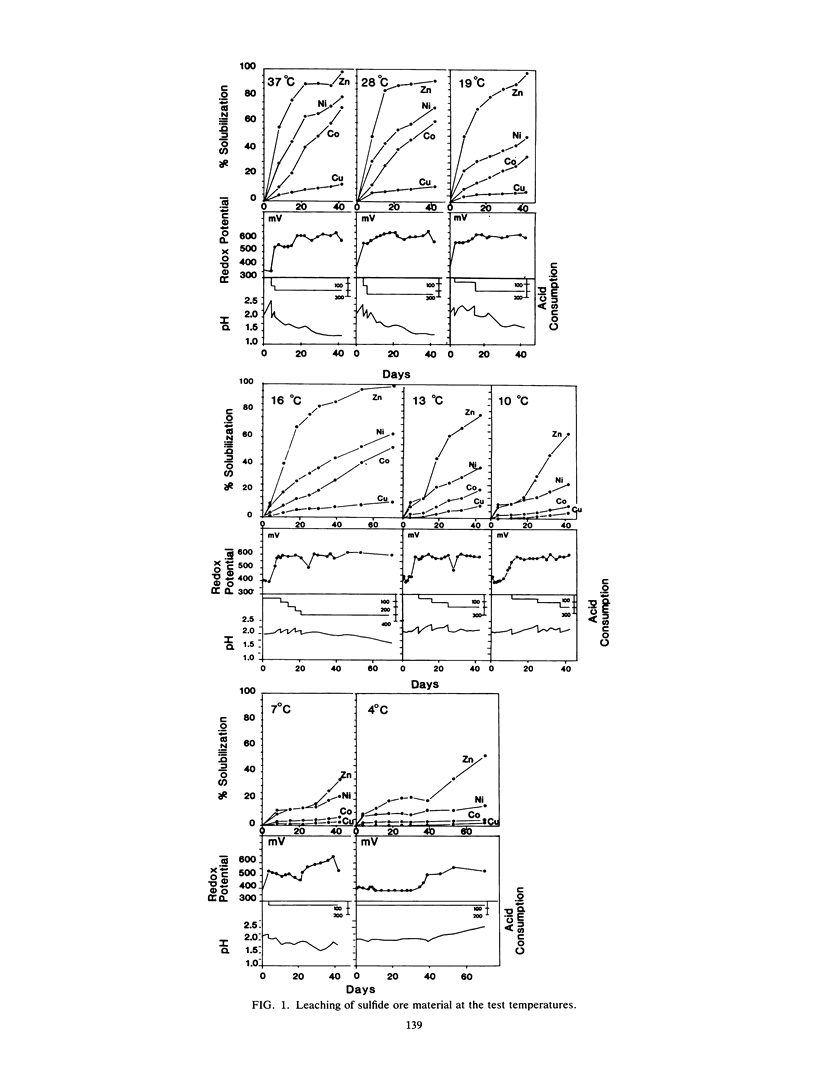
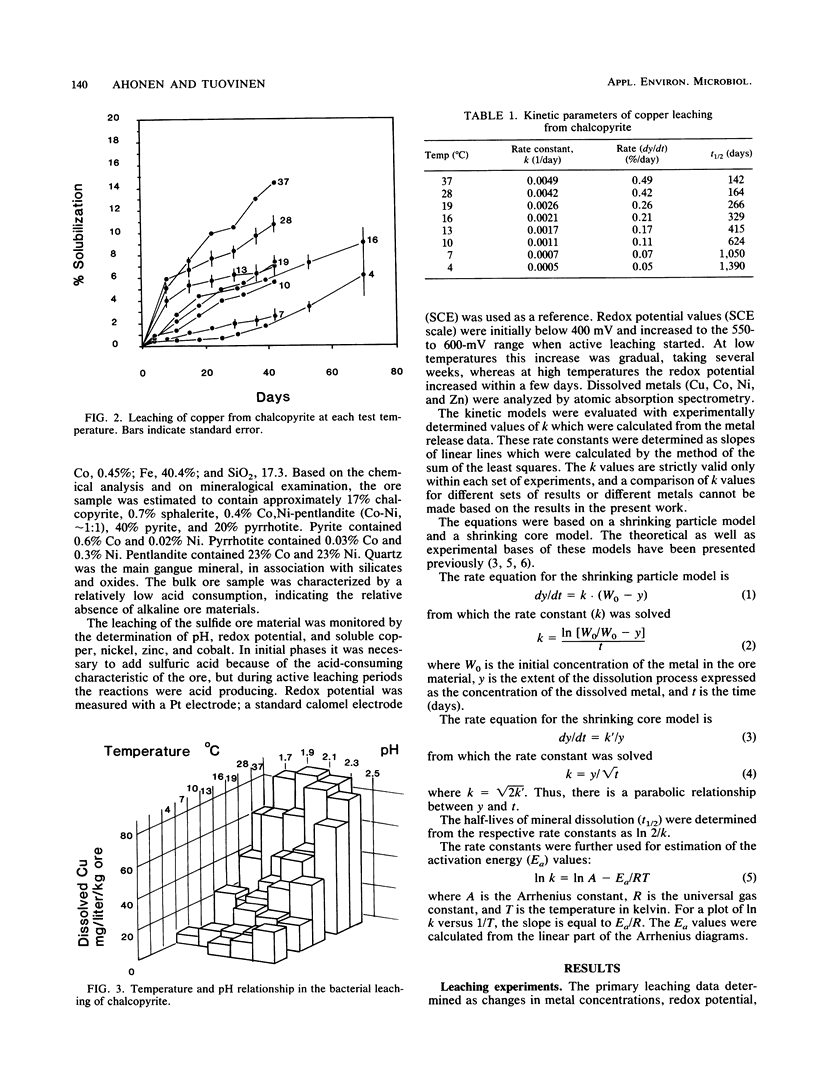
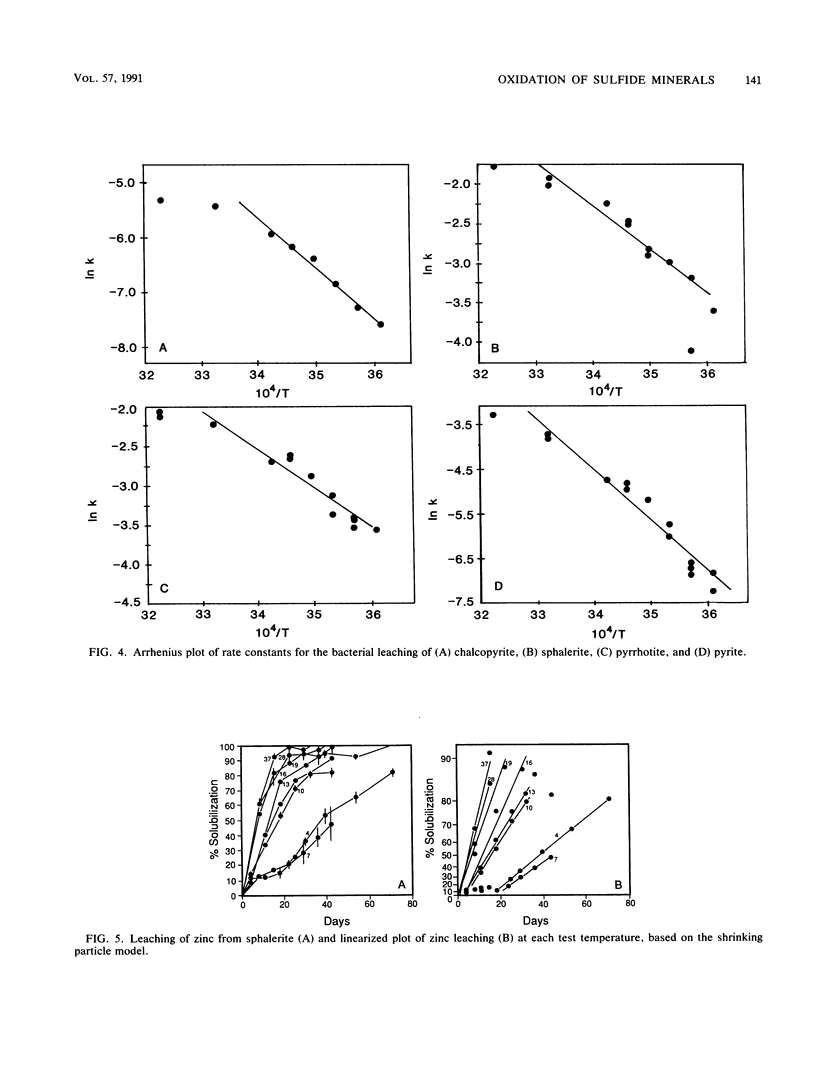
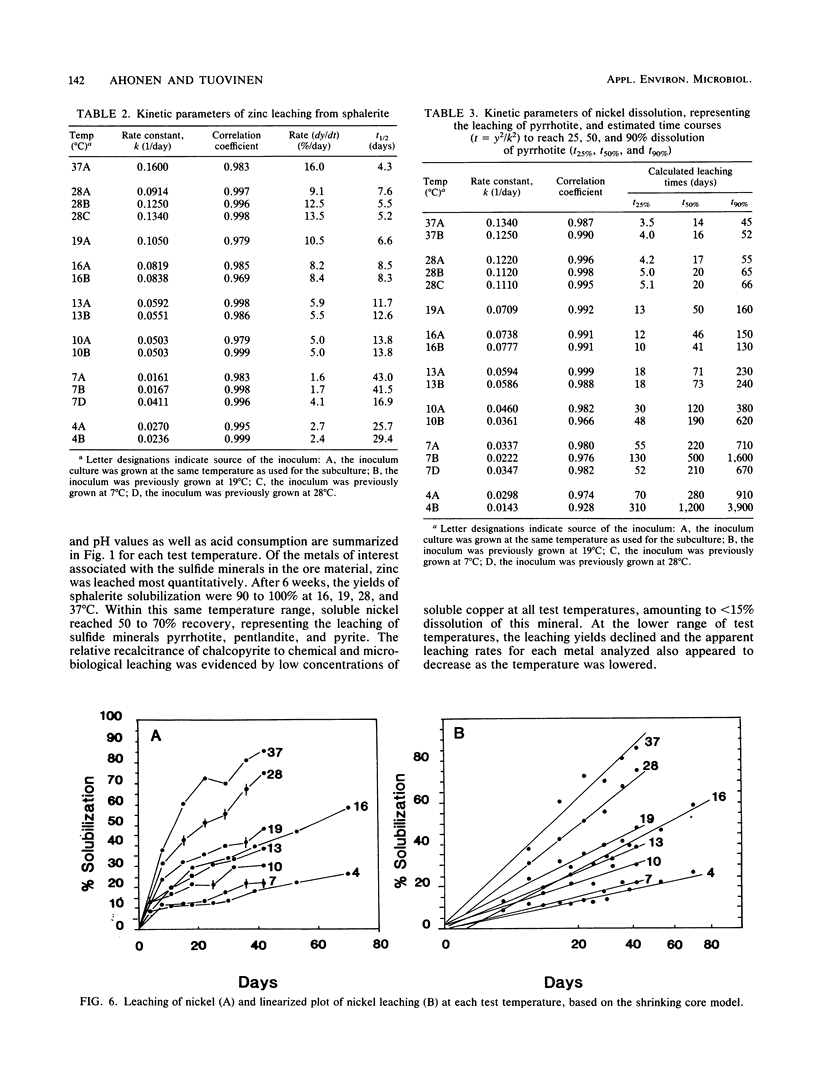
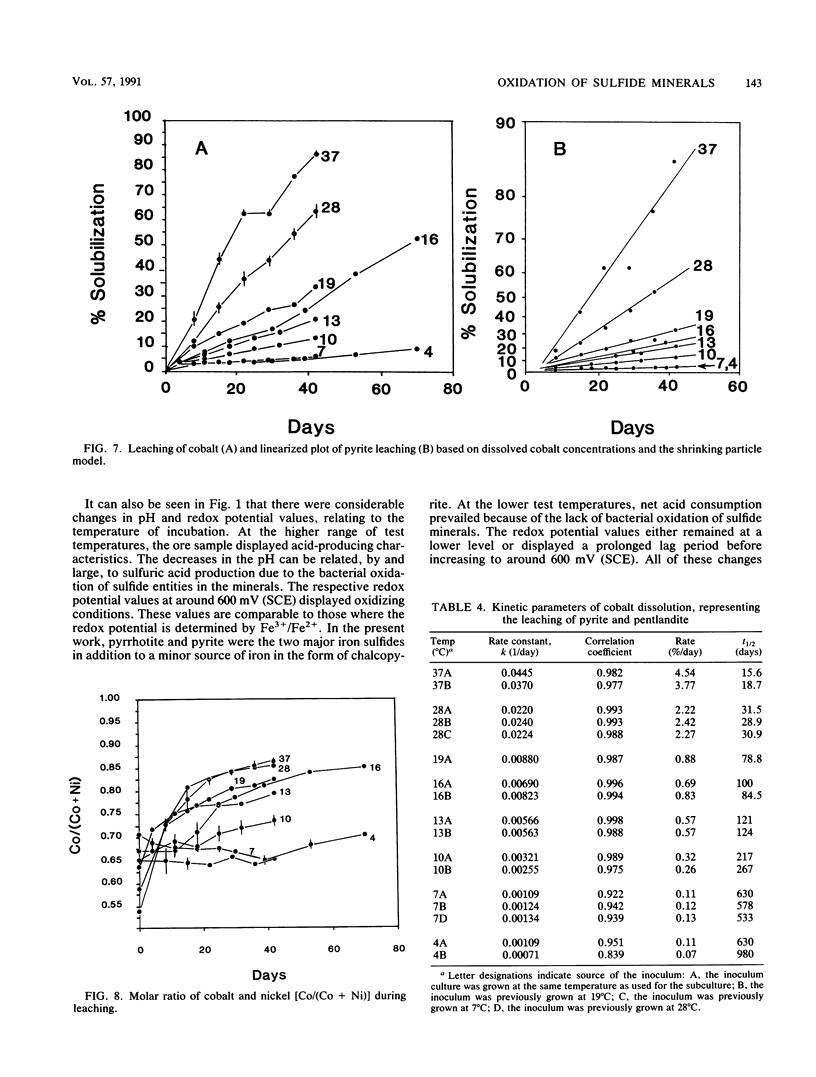
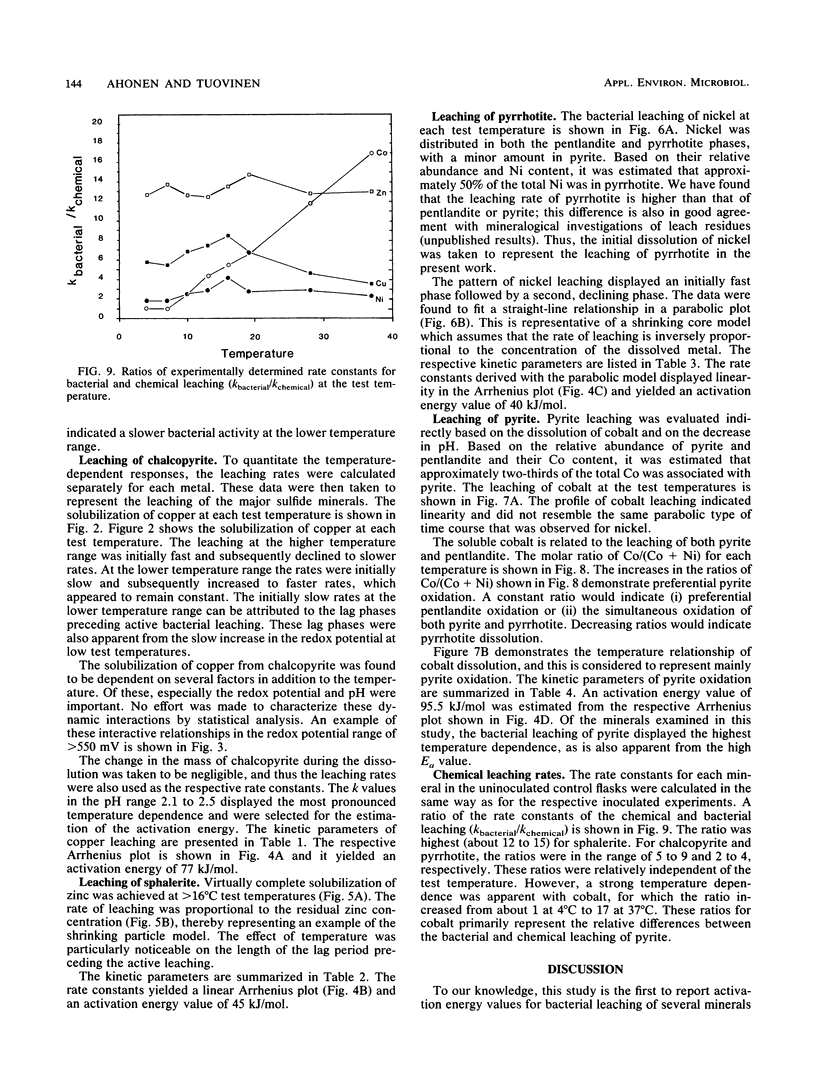
The microbiological leaching of a sulfide ore sample was investigated in shake flask experiments. The ore sample contained pyrite, pyrrhotite, pentlandite, sphalerite, and chalcopyrite as the main sulfide minerals. The tests were performed at eight different temperatures in the range of 4 to 37°C. The primary data were used for rate constant calculations, based on kinetic equations underlying two simplified models of leaching, i.e., a shrinking particle model and a shrinking core model. The rate constants thus derived were further used for the calculation of activation energy values for some of the sulfide minerals present in the ore sample. The chalcopyrite leaching rates were strongly influenced by the interaction of temperature, pH, and redox potential. Sphalerite leaching could be explained with the shrinking particle model. The data on pyrrhotite leaching displayed good fit with the shrinking core model. Pyrite leaching was found to agree with the shrinking particle model. Activation energies calculated from the rate of constants suggested that the rate-limiting steps were different for the sulfide minerals examined; they could be attributed to a chemical or biochemical reaction rather than to diffusion control.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahonen L., Tuovinen O. H. Kinetics of sulfur oxidation at suboptimal temperatures. Appl Environ Microbiol. 1990 Feb;56(2):560–562. doi: 10.1128/aem.56.2.560-562.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahonen L., Tuovinen O. H. Microbiological oxidation of ferrous iron at low temperatures. Appl Environ Microbiol. 1989 Feb;55(2):312–316. doi: 10.1128/aem.55.2.312-316.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


