Abstract
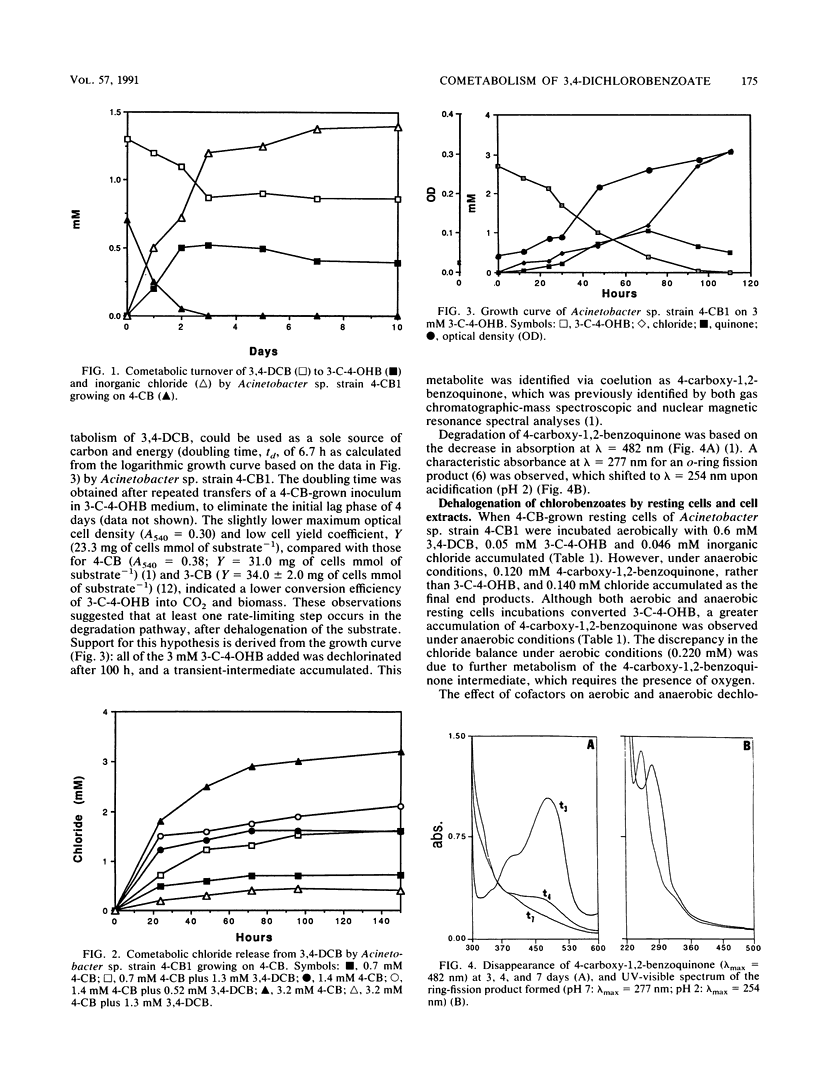
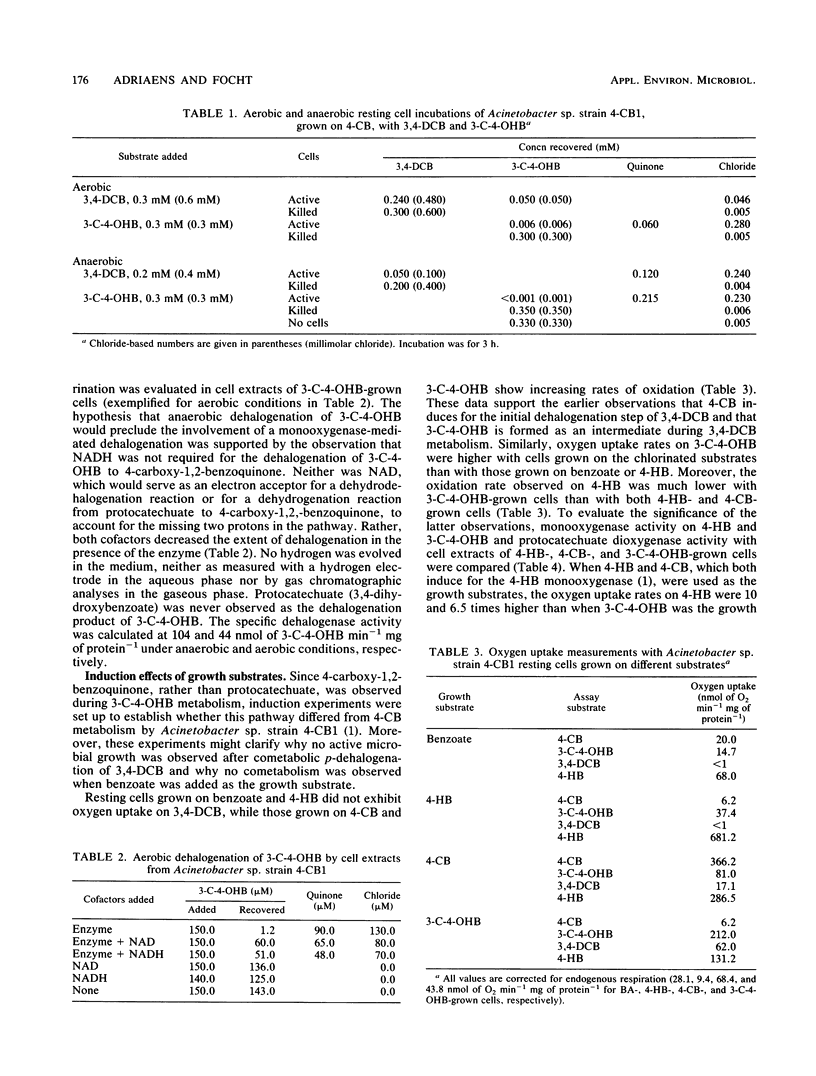
When Acinetobacter sp. strain 4-CB1 was grown on 4-chlorobenzoate (4-CB), it cometabolized 3,4-dichlorobenzoate (3,4-DCB) to 3-chloro-4-hydroxybenzoate (3-C-4-OHB), which could be used as a growth substrate. No cometabolism of 3,4-DCB was observed when Acinetobacter sp. strain 4-CB1 was grown on benzoate. 4-Carboxyl-1,2-benzoquinone was formed as an intermediate from 3,4-DCB and 3-C-4-OHB in aerobic and anaerobic resting-cell incubations and was the major transient intermediate found when cells were grown on 3-C-4-OHB. The first dechlorination step of 3,4-DCB was catalyzed by the 4-CB dehalogenase, while a soluble dehalogenase was responsible for dechlorination of 3-C-4-OHB. Both enzymes were inducible by the respective chlorinated substrates, as indicated by oxygen uptake experiments. The dehalogenase activity on 3-C-4-OHB, observed in crude cell extracts, was 109 and 44 nmol of 3-C-4-OHB min-1 mg of protein-1 under anaerobic and aerobic conditions, respectively. 3-Chloro-4-hydroxybenzoate served as a pseudosubstrate for the 4-hydroxybenzoate monooxygenase by effecting oxygen and NADH consumption without being hydroxylated. Contrary to 4-CB metabolism, the results suggest that 3-C-4-OHB was not metabolized via the protocatechuate pathway. Despite the ability of resting cells grown on 4-CB or 3-C-4-OHB to carry out all of the necessary steps for dehalogenation and catabolism of 3,4-DCB, it appeared that 3,4-DCB was unable to induce the necessary 4-CB dehalogenase for the initial p-dehalogenation step.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adriaens P., Kohler H. P., Kohler-Staub D., Focht D. D. Bacterial dehalogenation of chlorobenzoates and coculture biodegradation of 4,4'-dichlorobiphenyl. Appl Environ Microbiol. 1989 Apr;55(4):887–892. doi: 10.1128/aem.55.4.887-892.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed M., Focht D. D. Degradation of polychlorinated biphenyls by two species of Achromobacter. Can J Microbiol. 1973 Jan;19(1):47–52. doi: 10.1139/m73-007. [DOI] [PubMed] [Google Scholar]
- Benarde M. A., Koft B. W., Horvath R., Shaulis L. Microbial Degradation of the Sulfonate of Dodecyl Benzene Sulfonate. Appl Microbiol. 1965 Jan;13(1):103–105. doi: 10.1128/am.13.1.103-105.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- DAGLEY S., CHAPMAN P. J., GIBSON D. T., WOOD J. M. DEGRADATION OF THE BENZENE NUCLEUS BY BACTERIA. Nature. 1964 May 23;202:775–778. doi: 10.1038/202775a0. [DOI] [PubMed] [Google Scholar]
- DAGLEY S., EVANS W. C., RIBBONS D. W. New pathways in the oxidative metabolism of aromatic compounds by microorganisms. Nature. 1960 Nov 12;188:560–566. doi: 10.1038/188560a0. [DOI] [PubMed] [Google Scholar]
- Dorn E., Hellwig M., Reineke W., Knackmuss H. J. Isolation and characterization of a 3-chlorobenzoate degrading pseudomonad. Arch Microbiol. 1974;99(1):61–70. doi: 10.1007/BF00696222. [DOI] [PubMed] [Google Scholar]
- Dorn E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Substituent effects on 1,2-dioxygenation of catechol. Biochem J. 1978 Jul 15;174(1):85–94. doi: 10.1042/bj1740085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Two catechol 1,2-dioxygenases from a 3-chlorobenzoate-grown pseudomonad. Biochem J. 1978 Jul 15;174(1):73–84. doi: 10.1042/bj1740073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focht D. D., Shelton D. Growth kinetics of Pseudomonas alcaligenes C-0 relative to inoculation and 3-chlorobenzoate metabolism in soil. Appl Environ Microbiol. 1987 Aug;53(8):1846–1849. doi: 10.1128/aem.53.8.1846-1849.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Tomizuka N., Kamibayashi A. Effect of chlorine substitution on the bacterial metabolism of various polychlorinated biphenyls. Appl Environ Microbiol. 1979 Aug;38(2):301–310. doi: 10.1128/aem.38.2.301-310.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann J., Reineke W., Knackmuss H. J. Metabolism of 3-chloro-, 4-chloro-, and 3,5-dichlorobenzoate by a pseudomonad. Appl Environ Microbiol. 1979 Mar;37(3):421–428. doi: 10.1128/aem.37.3.421-428.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey W. J., Focht D. D. Degradation of mono-, di-, and trihalogenated benzoic acids by Pseudomonas aeruginosa JB2. Appl Environ Microbiol. 1990 Dec;56(12):3842–3850. doi: 10.1128/aem.56.12.3842-3850.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath R. S., Alexander M. Cometabolism of m-chlorobenzoate by an Arthrobacter. Appl Microbiol. 1970 Aug;20(2):254–258. doi: 10.1128/am.20.2.254-258.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath R. S. Cometabolism of the herbicide, 2,3,6-trichlorobenzoate by natural microbial populations. Bull Environ Contam Toxicol. 1972 May;7(5):273–276. doi: 10.1007/BF01684523. [DOI] [PubMed] [Google Scholar]
- Horvath R. S., Koft B. W. Degradation of alkyl benzene sulfonate by Pseudomonas species. Appl Microbiol. 1972 Feb;23(2):407–414. doi: 10.1128/am.23.2.407-414.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath R. S. Microbial co-metabolism and the degradation of organic compounds in nature. Bacteriol Rev. 1972 Jun;36(2):146–155. doi: 10.1128/br.36.2.146-155.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke D., Fritsche W. Nature and significance of microbial cometabolism of xenobiotics. J Basic Microbiol. 1985;25(9):603–619. doi: 10.1002/jobm.3620250910. [DOI] [PubMed] [Google Scholar]
- Kohler H. P., Kohler-Staub D., Focht D. D. Cometabolism of polychlorinated biphenyls: enhanced transformation of Aroclor 1254 by growing bacterial cells. Appl Environ Microbiol. 1988 Aug;54(8):1940–1945. doi: 10.1128/aem.54.8.1940-1945.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler H. P., Kohler-Staub D., Focht D. D. Degradation of 2-hydroxybiphenyl and 2,2'-dihydroxybiphenyl by Pseudomonas sp. strain HBP1. Appl Environ Microbiol. 1988 Nov;54(11):2683–2688. doi: 10.1128/aem.54.11.2683-2688.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröckel L., Focht D. D. Construction of chlorobenzene-utilizing recombinants by progenitive manifestation of a rare event. Appl Environ Microbiol. 1987 Oct;53(10):2470–2475. doi: 10.1128/aem.53.10.2470-2475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks T. S., Smith A. R., Quirk A. V. Degradation of 4-Chlorobenzoic Acid by Arthrobacter sp. Appl Environ Microbiol. 1984 Nov;48(5):1020–1025. doi: 10.1128/aem.48.5.1020-1025.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks T. S., Wait R., Smith A. R., Quirk A. V. The origin of the oxygen incorporated during the dehalogenation/hydroxylation of 4-chlorobenzoate by an Arthrobacter sp. Biochem Biophys Res Commun. 1984 Oct 30;124(2):669–674. doi: 10.1016/0006-291x(84)91607-3. [DOI] [PubMed] [Google Scholar]
- Mires M. H., Alexander C. H. The prophylactic treatment tuberculosis. Del Med J. 1972 Jul;44(7):187–190. [PubMed] [Google Scholar]
- Müller R., Thiele J., Klages U., Lingens F. Incorporation of [18O]water into 4-hydroxybenzoic acid in the reaction of 4-chlorobenzoate dehalogenase from pseudomonas spec. CBS 3. Biochem Biophys Res Commun. 1984 Oct 15;124(1):178–182. doi: 10.1016/0006-291x(84)90933-1. [DOI] [PubMed] [Google Scholar]
- Ohta Y., Higgins I., Ribbons D. W. Metabolism of resorcinylic compounds by bacteria. Purification and properties of orcinol hydroxylase from Pseudomonas putida 01. J Biol Chem. 1975 May 25;250(10):3814–3825. [PubMed] [Google Scholar]
- Ribbons D. W., Ohta Y. Uncoupling of electron transport from oxygenation in the mono-oxygenase, orcinol hydroxylase. FEBS Lett. 1970 Dec 28;12(2):105–108. doi: 10.1016/0014-5793(70)80574-9. [DOI] [PubMed] [Google Scholar]
- Schreiber A., Hellwig M., Dorn E., Reineke W., Knackmuss H. J. Critical Reactions in Fluorobenzoic Acid Degradation by Pseudomonas sp. B13. Appl Environ Microbiol. 1980 Jan;39(1):58–67. doi: 10.1128/aem.39.1.58-67.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White-Stevens R. H., Kamin H. Studies of a flavoprotein, salicylate hydroxylase. I. Preparation, properties, and the uncoupling of oxygen reduction from hydroxylation. J Biol Chem. 1972 Apr 25;247(8):2358–2370. [PubMed] [Google Scholar]
- Zaitsev G. M., Baskunov B. P. Utilizatsiia 3-khlorbenzoinoi kisloty Acinetobacter calcoaceticus. Mikrobiologiia. 1985 Mar-Apr;54(2):203–208. [PubMed] [Google Scholar]



